Abstract
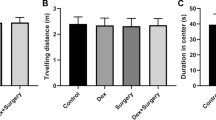
Postoperative cognitive dysfunction (POCD) is recognized as a complication after surgery in the elderly. The exact pathogenic mechanisms of POCD are still unknown. In this study, we investigated the role of iron accumulation within the central nervous system in the development of cognitive dysfunction in rats following splenectomy. Cognitive function was assessed using a Morris water maze on postoperative days 1, 3, and 7. Impaired cognitive function was observed on days 1 and 3 after splenectomy, while an anesthesia-alone group showed no significant difference from the control. Serum iron levels decreased and brain iron content increased on days 1 and 3 after surgery, which was in parallel with the impairment of cognitive function. Furthermore, the levels of proteins involved in the maintenance of brain iron homeostasis, including ferritin, transferrin receptor 1, and iron regulatory protein 2, were significantly different at postoperative days 1 and 3 in the hippocampus of splenectomized animals when compared with those of the control. The alterations in iron homeostasis were accompanied by intensified oxidative stress as measured by increases in the lipid peroxidation product, malondialdehyde, and a decrease in the levels of superoxide dismutase activity. Overall, these findings suggest that the impaired cognitive function was primarily due to surgical trauma rather than anesthesia. Increased iron accumulation and oxidative stress in the brain, especially in the hippocampus, may be involved in the pathogenesis of POCD.



Similar content being viewed by others
References
Riis J, Lomholt B, Haxholdt O, Kehlet H, Valentin N, Danielsen U, Dyrberg V (1983) Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand 27(1):44–49
Deiner S, Silverstein JH (2009) Postoperative delirium and cognitive dysfunction. Br J Anaesth 103(Suppl 1):i41–i46
Martin JF, Melo RO, Sousa LP (2008) Postoperative cognitive dysfunction after cardiac surgery. Rev Bras Cir Cardiovasc 23(2):245–255
Newman S, Stygall J, Hirani S, Shaefi S, Maze M (2007) Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology 106(3):572–590
Ramaiah R, Lam AM (2009) Postoperative cognitive dysfunction in the elderly. Anesthesiol Clin 27(3):485–496
Sauër AM, Kalkman C, van Dijk D (2009) Postoperative cognitive decline. J Anesth 23(2):256–259
Krenk L, Rasmussen LS, Kehlet H (2010) New insights into the pathophysiology of postoperative cognitive dysfunction. Acta anaesthesiologica Scandinavica 54(8):951–956
Stankiewicz JM, Brass SD (2009) Role of iron in neurotoxicity: a cause for concern in the elderly? Curr Opin Clin Nutr Metab Care 12(1):22–29
Ma L, Wang W, Zhao M, Li M (2008) Foot-shock stress-induced regional iron accumulation and altered iron homeostatic mechanisms in rat brain. Biol Trace Elem Res 126(1–3):204–213
Ke Y, Qian ZM (2007) Brain iron metabolism: neurobiology and neurochemistry. Prog Neurobiol 83(3):149–173
Ke Y, Ming Qian Z (2003) Iron misregulation in the brain: a primary cause of neurodegenerative disorders. Lancet Neurol 2(4):246–253
Dröge W, Schipper HM (2007) Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell 6(3):361–370
Stankiewicz J, Panter SS, Neema M, Arora A, Batt CE, Bakshi R (2007) Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics 4(3):371–386
Smith MA, Zhu X, Tabaton M, Liu G, McKeel DW Jr, Cohen ML, Wang X, Siedlak SL, Dwyer BE, Hayashi T, Nakamura M, Nunomura A, Perry G (2010) Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis 19(1):363–372
LeVine SM (1997) Iron deposits in multiple sclerosis and Alzheimer’s disease brains. Brain Res 760(1–2):298–303
Smith MA, Harris PL, Sayre LM, Perry G (1997) Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci USA 94(18):9866–9868
Clark TA, Lee HP, Rolston RK, Zhu X, Marlatt MW, Castellani RJ, Nunomura A, Casadesus G, Smith MA, Lee HG, Perry G (2010) Oxidative stress and its implications for future treatments and management of Alzheimer disease. Int J Biomed Sci 6(3):225–227
Baranov D, Bickler PE, Crosby GJ et al (2009) Consensus statement: first international workshop on anesthetics and Alzheimer’s disease. Anesth Analg 108:1627–1630
Mandal PK, Pettegrew JW, McKeag DW, Mandal R (2006) Alzheimer’s disease: halothane induces Abeta peptide to oligomeric form–solution NMR studies. Neurochem Res 31(7):883–890
Alves HN, da Silva AL, Olsson IA, Orden JM, Antunes LM (2010) Anesthesia with intraperitoneal propofol, medetomidine, and fentanyl in rats. J Am Assoc Lab Anim Sci 49(4):454–459
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2):848–858
Focht SJ, Snyder BS, Beard JL, Van Gelder W, Williams LR, Connor JR (1997) Regional distribution of iron, transferrin, ferritin, and oxidatively-modified proteins in young and aged Fischer 344 rat brains. Neuroscience 79(1):255–261
Nikolova-Todorova Z, Troić T (2003) Effect of surgical trauma on patient nutritional status. Med Arh 57(4 Suppl 1):29–31
Rosczyk HA, Sparkman NL, Johnson RW (2008) Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol 43(9):840–846
Cao XZ, Ma H, Wang JK, Liu F, Wu BY, Tian AY, Wang LL, Tan WF (2010) Postoperative cognitive deficits and neuroinflammation in the hippocampus triggered by surgical trauma are exacerbated in aged rats. Prog Neuropsychopharmacol Biol Psychiatry 34(8):1426–1432
Jones MJ (1998) Influence of anesthetic methods on mental function. Acta Chir Scand 550:169–176
Wu CL, Hsu W, Richman JM, Raja SN (2004) Postoperative cognitive function as an outcome of regional anesthesia and analgesia. Reg Anesth Pain Med 29(3):257–268
Antila H, Salo M (1990) Serum iron, zinc, copper, selenium, and bromide concentrations after coronary bypass operation. JPEN J Parenter Enteral Nutr 14(1):85–89
Marques F, Falcao AM, Sousa JC, Coppola G, Geschwind D, Sousa N, Correia-Neves M, Palha JA (2009) Altered iron metabolism is part of the choroid plexus response to peripheral inflammation. Endocrinology 150(6):2822–2828
Lyle RM, Weaver CM, Sedlock DA, Rajaram S, Martin B, Melby CL (1992) Iron status in exercising woman: the effect of oral iron therapy vs increased consumption of muscle foods. Am J Clin Nutr 56(6):1049–1055
Brewer GJ (2007) Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer’s disease. Exp Biol Med (Maywood) 232(2):323–335
Bartzokis G, Sultzer D, Cummings J, Holt LE, Hance DB, Henderson VW, Mintz J (2000) In vivo evaluation of brain iron in Alzheimer disease using magnetic resonance imaging. Arch Gen Psychiatry 57(1):47–53
Schrag M, Mueller C, Oyoyo U, Smith MA, Kirsch WM (2011) Iron, zinc and copper in the Alzheimer’s disease brain: a quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Prog Neurobiol 94(3):296–306
Park UJ, Lee YA, Won SM, Lee JH, Kang SH, Springer JE, Lee YB, Gwag BJ (2011) Blood-derived iron mediates free radical production and neuronal death in the hippocampal CA1 area following transient forebrain ischemia in rat. Acta Neuropathol 121(4):459–473
Cipolla MJ, Godfrey JA, Wiegman MJ (2009) The effect of ovariectomy and estrogen on penetrating brain arterioles and blood–brain barrier permeability. Microcirculation 16(8):685–693
Okamura T, Ishibashi N, Zurakowski D, Jonas RA (2010) Cardiopulmonary bypass increases permeability of the blood cerebrospinal fluid barrier. Ann Thorac Surg 89(1):187–194
Qian ZM, Shen X (2001) Brain iron transport and neurodegeneration. Trends Mol Med 7(3):103–108
Pantopoulos K (2004) Iron metabolism and the IRE/IRP regulator system: an update. Ann NY Acad Sci 1012:1–13
Pinero DJ, Hu J, Connor JR (2000) Alterations in the interaction between iron regulatory proteins and their iron responsive element in normal and Alzheimer’s diseased brains. Cell Mol Biol 46(4):761–776
Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, Smith MA (2010) Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology 59(4–5):290–294
Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M (2007) Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology 106(3):436–443
Acknowledgments
We thank Prof. Shu-Ren Wang and Dr. Xiao-Li Yang for their kind and skillful technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
An, LN., Yue, Y., Guo, WZ. et al. Surgical Trauma Induces Iron Accumulation and Oxidative Stress in a Rodent Model of Postoperative Cognitive Dysfunction. Biol Trace Elem Res 151, 277–283 (2013). https://doi.org/10.1007/s12011-012-9564-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9564-9