Abstract
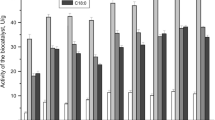
The tunable textural properties of self-oriented mesoporous silica were investigated for their suitability as enzyme immobilization matrices to support transesterification of rice bran oil. Different morphologies of mesoporous silica (rod-like, rice-like, and spherical) were synthesized and characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and nitrogen adsorption–desorption isotherms. The surface area, pore size, and ordered arrangement of the pores were found to influence the immobilization and activity of the enzyme in the mesopores. The immobilization in rod-like silica was highest with an immobilization efficiency of 63 % and exhibited minimal activity loss after immobilization. Functionalization of the mesoporous surface with ethyl groups further enhanced the enzyme immobilization. The free enzyme lost most of its activity at 50 °C while the immobilized enzyme showed activity even up to 60 °C. Transesterified product yield of nearly 82 % was obtained for 24 h of reaction with enzyme immobilized on ethyl-functionalized SBA-15 at an oil:methanol ratio of 1:3. Fourier transform infrared spectroscopy (FT–IR) and Gas chromatography–mass spectrometry (GC-MS) were used to characterize the transesterified product obtained. The reusability of the immobilized enzyme was studied for 3 cycles.








Similar content being viewed by others
References
Ye, P., Jiang, J., & Xu, Z. K. (2007). Adsorption and activity of lipase from Candida rugosa on the chitosan-modified poly(acrylonitrile-co-maleic acid) membrane surface. Colloids and Surfaces B: Biointerfaces, 60, 62–67.
Dizge, N., Aydiner, C., Imer, D. Y., Bayramoglu, M., Tanriseven, A., & Keskinler, B. (2009). Biodiesel production from sunflower, soybean, and waste cooking oils by transesterification using lipase immobilized onto a novel microporous polymer. Bioresource Technology, 100, 1983–1991.
Prlainovic, N. Z., Knezevic-Jugovic, Z. D., Mijin, D. Z., & Bezbradica, D. I. (2011). Immobilization of lipase from Candida rugosa on Sepabeads((R)): the effect of lipase oxidation by periodates. Bioprocess and Biosystems Engineering, 34, 803–810.
Bordes, F., Barbe, S., Escalier, P., Mourey, L., Andre, I., Marty, A., & Tranier, S. (2010). Exploring the conformational states and rearrangements of Yarrowia lipolytica Lipase. Biophysical Journal, 99, 2225–2234.
Piamtongkam, R., Duquesne, S., Bordes, F., Barbe, S., Andre, I., Marty, A., & Chulalaksananukul, W. (2011). Enantioselectivity of Candida rugosa lipases (Lip1, Lip3, and Lip4) towards 2-bromo phenylacetic acid octyl esters controlled by a single amino acid. Biotechnology and Bioengineering, 108, 1749–1756.
Gao, S., Wang, Y., Diao, X., Luo, G., & Dai, Y. (2010). Effect of pore diameter and cross-linking method on the immobilization efficiency of Candida rugosa lipase in SBA-15. Bioresource Technology, 101, 3830–3837.
Yin, P., Chen, W., Liu, W., Chen, H., Qu, R., Liu, X., Tang, Q., & Xu, Q. (2013). Efficient bifunctional catalyst lipase/organophosphonic acid-functionalized silica for biodiesel synthesis by esterification of oleic acid with ethanol. Bioresource Technology, 140, 146–151.
Grasset, L., Cordier, D., & Ville, A. (1977). Woven silk as a carrier for the immobilization of enzymes. Biotechnology and Bioengineering, 19, 611–618.
Mateo, C., Grazu, V., Pessela, B. C., Montes, T., Palomo, J. M., Torres, R., Lopez-Gallego, F., Fernandez-Lafuente, R., & Guisan, J. M. (2007). Advances in the design of new epoxy supports for enzyme immobilization-stabilization. Biochemical Society Transactions, 35, 1593–1601.
Bayramoglu, G., Altintas, B., & Arica, M. Y. (2011). Reversible immobilization of uricase on conductive polyaniline brushes grafted on polyacrylonitrile film. Bioprocess and Biosystems Engineering, 34, 127–134.
Ducker, R. E., Montague, M. T., & Leggett, G. J. (2008). A comparative investigation of methods for protein immobilization on self-assembled monolayers using glutaraldehyde, carbodiimide, and anhydride reagents. Biointerphases, 3, 59–65.
Daglioglu, C., & Zihnioglu, F. (2012). Covalent immobilization of trypsin on glutaraldehyde-activated silica for protein fragmentation. Artificial Cells, Blood Substitutes, and Immobilization Biotechnology, 40, 378–384.
Ferrarotti, S. A., Bolivar, J. M., Mateo, C., Wilson, L., Guisan, J. M., & Fernandez-Lafuente, R. (2006). Immobilization and stabilization of a cyclodextrin glycosyltransferase by covalent attachment on highly activated glyoxyl-agarose supports. Biotechnology Progress, 22, 1140–1145.
Vinoba, M., Bhagiyalakshmi, M., Jeong, S. K., Yoon, Y. I., & Nam, S. C. (2012). Immobilization of carbonic anhydrase on spherical SBA-15 for hydration and sequestration of CO2. Colloids and Surfaces B: Biointerfaces, 90, 91–96.
Li, S., Wu, Z., Lu, M., Wang, Z., & Li, Z. (2013). Improvement of the enzyme performance of trypsin via adsorption in mesoporous silica SBA-15: hydrolysis of BAPNA. Molecules, 18, 1138–1149.
Wan, M. M., Lin, W. G., Gao, L., Gu, H. C., & Zhu, J. H. (2012). Promoting immobilization and catalytic activity of horseradish peroxidase on mesoporous silica through template micelles. Journal of Colloid and Interface Science, 377, 497–503.
Magner, E. (2013). Immobilisation of enzymes on mesoporous silicate materials. Chemical Society Reviews, 42, 6213–6222.
Gandhi, S., Sethraman, S., & Krishnan, U. M. (2010). Influence of polyhydric solvents on the catalytic and adsorption properties of self-oriented mesoporous SBA-15 silica. Journal of Porous Materials, 18, 329–336.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Hiol, A., Jonzo, M. D., Rugani, N., Druet, D., Sarda, L., & Comeau, L. C. (2000). Purification and characterization of an extracellular lipase from a thermophilic Rhizopus oryzae strain isolated from palm fruit. Enzyme and Microbial Technology, 26, 421–430.
Bayramoglu, G., Karagoz, B., Altintas, B., Arica, M. Y., & Bicak, N. (2011). Poly(styrene-divinylbenzene) beads surface functionalized with di-block polymer grafting and multi-modal ligand attachment: performance of reversibly immobilized lipase in ester synthesis. Bioprocess and Biosystems Engineering, 34, 735–746.
Balcao, V. M., & Malcata, F. X. (1998). On the performance of a hollow-fiber bioreactor for acidolysis catalyzed by immobilized lipase. Biotechnology and Bioengineering, 60, 114–123.
Acknowledgments
The authors wish to acknowledge the characterization facilities established from the PG Teaching fund of the Nanomission council, Department of Science and Technology, and infrastructure support from SASTRA University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Prashanth Ramachandran and Guru Krupa Narayanan have equal contribution to this study.
Rights and permissions
About this article
Cite this article
Ramachandran, P., Narayanan, G.K., Gandhi, S. et al. Rhizopus oryzae Lipase Immobilized on Hierarchical Mesoporous Silica Supports for Transesterification of Rice Bran Oil. Appl Biochem Biotechnol 175, 2332–2346 (2015). https://doi.org/10.1007/s12010-014-1432-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1432-y