Abstract
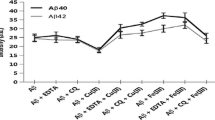
The toxic effect of Aβ42 induced by copper nanoparticle (Cu NPs) was studied by atomic force microscopy (AFM), circular dichroism (CD) spectroscopy, and Thioflavin T (ThT) fluorescence technique. Five hundred nanometers of copper nanoparticle capped with polyvinylpyrrolidone (PVP) was used to evaluate the aggregation and fibrils of Aβ42. The morphologies of Aβ42 incubated in the presence of Cu NPs changed gradually. The aggregation and fibrils were observed in AFM images. However, in the presence of polysaccharides, the Cu NPs-induced fibrillation of Aβ42 was inhibited. Interestingly, the formed Cu NPs–polysaccharides complexes can even remodel the preformed Aβ42 fibrils into the low neurotoxic amorphous aggregates, which were maybe ascribed to the higher affinity of polysaccharides for Aβ42 than Cu NPs. Besides, it was found that the binding constant of Cu NPs to Aβ42 is smaller than that of polysaccharides. The relationship among polysaccharides, copper nanoparticle, and Aβ42 morphologies and its neurotoxicity were discussed, and the binding force was analyzed.





Similar content being viewed by others
References
Barnham, K. J., Smith, D. G., & Cappai, R. (2007). The redox chemistry of the Alzheimer’s disease amyloid beta peptide. Biochimica et Biophysica Acta - Biomembranes, 1768, 1976–1990.
Lin, Y. F., Huang, M. C., & Liu, H. C. (2013). Glycogen synthase kinase 3β gene polymorphisms may be associated with bipolar I disorder and the therapeutic response tolithium. Journal of Affective Disorders, 147(1–3), 401–406.
Brown, A. M., Lemkul, J. A., Schaum, N., & Bevan, D. R. (2014). Simulations of monomeric amyloid β-peptide (1–40) with varying solution conditions and oxidation state of Met35: implications for aggregation. Archives of Biochemistry and Biophysics, 545, 44–52.
Biswajoy, B., Subrata, K., Sumit, K. D., Suman, B., Roy, D., Mukhopadhyay, T. K., Das, S., & Nand, P. (2013). In situ synthesis and antibacterial activity of copper nanoparticle loaded natural montmorillonite clay based on contact inhibition and ion release. Colloids and Surfaces B: Biointerfaces, 108, 358–365.
Torres-Vega, J. J., Medrano, L. R., Landauro, C. V., & Rojas-Tapia, J. (2014). Determination of the threshold of nanoparticle behavior: structural and electronic properties study of nano-sized copper. Physica B, 436, 74–79.
Chen, Y., Wang, D., Zhu, X., Zheng, X., & Feng, L. (2012). Long term effects of copper nanoparticles on wastewater biological nutrient removal and N2O generation in the activated sludge process. Environmental Science & Technology, 46, 12452–12458.
Maria, V. L., & Bebianno, M. J. (2011). Antioxidant and lipid peroxidation responses inMytilus galloprovincialis exposed to mixtures of benzo(a)pyrene and copper. Comparative Biochemistry and Physiology - Part C, 154, 56–63.
Griffitt, R. J., Weil, R., Hyndman, K. A., Denslow, N. D., Powers, K., Taylor, D., & Barber, D. S. (2007). Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environmental Science & Technology, 41, 8178–8186.
Chen, Z., Meng, H., Xing, G., Chen, C., Zhao, Y., Jia, G., Wang, T., Yuan, H., Ye, C., Zhao, F., Chai, Z., Zhu, C., Fang, X., Ma, B., & Wan, L. (2006). Acute toxicological effects of copper nanoparticles in vivo. Toxicology Letters, 163, 109–120.
Prabhu, B. M., Ali, S. F., Murdock, R. C., Hussain, S. M., & Srivatsan, M. (2010). Copper nanoparticles exert size and concentration dependent toxicity on somatosensory neurons of rat. Nanotoxicology, 04, 150–160.
Zatta, P., Ricchelli, F., Drago, D., Filippi, B., & Tognon, G. (2005). Aluminum-triggered structural modifications and aggregation of beta-amyloids. Cellular and Molecular Life Sciences, 62, 1724–1733.
Exley, C. (2006). Aluminium and iron, but neither copper nor zinc, are key to the precipitation of beta-sheets of A beta (42) in senile plaque cores in Alzheimer’s disease. Journal of Alzheimer’s Disease, 10, 173–177.
Dong, S. W., Gao, Z. H., Shen, X. Y., Xue, H. W., & Li, X. (2014). Comparative proteomic analysis shows an elevation of Mdh1 associated with hepatotoxicity induced by copper nanoparticle in rats. Journal of Integrative Agriculture, 13(05), 1073–1081.
Karlsson, A. L., Cronholm, P., Hedberg, Y., Tornberg, M., Battice, L. D., Svedhem, S., & Odnevall, I. (2013). Cell membrane damage and protein interaction induced by copper containing nanoparticles—importance of the metal release process. Toxicology, 313(01), 59–69.
Sahin, N. O., & Burgess, D. J. (2003). Competitive interfacial adsorption of blood proteins. Farmaco, 58, 1017–1021.
Song, G. L., & Du, Q. Z. (2010). Isolation of a polysaccharide with anticancer activity from Auricularia polytricha using high-speed countercurrent chromatography with an aqueous two-phase system. Journal of Chromatography A, 1217, 5930–5934.
Xiao, Q., Huang, S., Qi, Z. D., Zhou, B., He, Z. K., & Liu, Y. (2008). Conformation, thermodynamics and stoichiometry of HSA adsorbed to colloidal CdSe/ZnS quantum dots. Biochimica et Biophysica Acta - Proteins & Proteomics, 1487, 1020–1027.
Biancalana, M., & Koide, S. (1804). Molecular mechanism of thioflavin-T binding to amyloid fibrils. Biochimica et Biophysica Acta - Proteins & Proteomics, 2010, 1405–1412.
Krebs, M. R. H., Bromley, E. H. C., & Donald, A. M. (2005). The binding of thioflavin-T to amyloid fibrils: localisation and implications. Journal of Structural Biology, 149, 30–37.
Acknowledgments
We gratefully acknowledge the financial support of Scientific Research Foundation of Minnan Normal University (L20629) and Key Research Items of Fujian Province (2012N0031).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Zhang, G. & Zou, J. The Aggregation of Aβ42 Induced by Nano Copper and the Antagonistic Action of Polysaccharides. Appl Biochem Biotechnol 175, 1557–1566 (2015). https://doi.org/10.1007/s12010-014-1385-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1385-1