Abstract
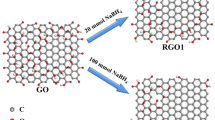
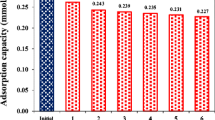
In this study, the effects of bio-reduced graphene oxide (BRGO) on the bio-reduction of Acid Red 18 (AR 18) by Shewanella algae were first investigated, and a possible mechanism of BRGO-mediated AR 18 bio-decolorization was proposed. The prepared BRGO was characterized by X-ray photoelectron spectroscopy (XPS), X-ray diffractometer (XRD), infrared spectroscopy (IR), Raman spectra, and transmission electron microscope (TEM), respectively. Moreover, electrochemical experiment demonstrated that BRGO is of good electrical conductivity. AR 18 bio-decolorization could be enhanced in dose-dependent manner of BRGO. The maximum increase in AR 18 removal efficiency was observed at a dose of 0.075 g L−1 BRGO. Under the same conditions, BRGO could also improve the decolorization rates of Acid Red 88, Acid Red 27, and Acid Red 73. During decolorization, the formation of BRGO and cells composite was observed, which is beneficial for transferring electrons from cells to BRGO. In addition, BRGO could accelerate the bio-decolorization of AR 18 under saline conditions (2–7 %). These findings indicate that BRGO can accelerate the electrons transfer from cells to azo dyes.







Similar content being viewed by others
References
Allen, M. J., Tung, V. C., & Kaner, R. B. (2009). Honeycomb carbon: a review of graphene. Chemical Reviews, 110, 132–145.
Geim, A. K. (2009). Graphene: status and prospects. Science, 324, 1530–1534.
Rao, C. N. R., Sood, A. K., Subrahmanyam, K. S., & Govindaraj, A. (2009). Graphene: the new two-dimensional nanomaterial. Angewandte Chemie, International Edition, 48, 7752–7777.
Chen, W., Duan, L., & Zhu, D. (2007). Adsorption of polar and nonpolar organic chemicals to carbon nanotubes. Environmental Science & Technology, 41, 8295–8300.
Pan, B., & Xing, B. (2008). Adsorption mechanisms of organic chemicals on carbon nanotubes. Environmental Science & Technology, 42, 9005–9013.
Fu, H., & Zhu, D. (2013). Graphene oxide-facilitated reduction of nitrobenzene in sulfide-containing aqueous solutions. Environmental Science & Technology, 47, 4204–4210.
Gurunathan, S., Han, J. W., Eppakayala, V., & Kim, J. H. (2013). Microbial reduction of graphene oxide by Escherichia coli: a green chemistry approach. Colloids and Surfaces. B, Biointerfaces, 102, 772–777.
Saratale, R. G., & Saratale, G. D. (2011). Bacterial decolorization and degradation of azo dyes: a review. Journal Taiwan Institute Chemical Engineers, 42, 138–157.
Pandey, A., Singh, P., & Iyengar, L. (2007). Bacterial decolorization and degradation of azo dyes. International Biodeterioration & Biodegradation, 59, 73–84.
Stolz, A. (2001). Basic and applied aspects in the microbial degradation of azo dyes. Applied Microbiology and Biotechnology, 56, 69–80.
Dos Santos, A. B., Cervantes, F. J., & Van Lier, J. B. (2007). Review paper on current technologies for decolourisation of textile wastewaters: perspectives for anaerobic biotechnology. Bioresource Technology, 98, 2369–2385.
Emilia Rios-Del Toro, E., Celis, L. B., Cervantes, F. J., & Rangel-Mendez, J. R. (2013). Enhanced microbial decolorization of methyl red with oxidized carbon fiber as redox mediator. Journal of Hazardous Materials, 260, 967–974.
Pereira, L., Pereira, R., Pereira, M. F. R., Van der Zee, F. P., Cervantes, F. J., & Alves, M. M. (2010). Thermal modification of activated carbon surface chemistry improves its capacity as redox mediator for azo dye reduction. Journal of Hazardous Materials, 183, 931–939.
Shen, W., Li, Z., & Liu, Y. (2008). Surface chemical functional groups modification of porous carbon. Recent Patents on Chemical Engineering, 1, 27–40.
Khanra, P., Kuila, T., Kim, N. H., Bae, S. H., Yu, D., & Lee, J. H. (2012). Simultaneous biofunctionalization and reduction of graphene oxide by baker’s yeast. Chemical Engineering Journal, 183, 526–533.
Kaniyoor, A., Baby, T. T., & Ramaprabhu, S. (2010). Graphene synthesis via hydrogen induced low temperature exfoliation of graphiteoxide. Journal of Materials Chemistry, 20, 8467–8469.
Dreyer, D. R., Park, S., Bielawski, C., & Ruoff, R. S. (2010). The chemistry of graphene oxide. Chemical Society Reviews, 39, 228.
Yuan, Y., Zhou, S. G., Zhao, B., Zhuang, L., & Wang, Y. Q. (2012). Microbially-reduced graphene scaffolds to facilitate extracellular electron transfer in microbial fuel cells. Bioresource Technology, 116, 453–458.
Brutinel, E. D., & Gralnick, J. A. (2012). Shuttling happens: soluble flavin mediators of extracellular electron transfer in Shewanella. Applied Microbiology and Biotechnology, 91, 43–48.
Field, J. A., & Brady, J. (2003). Riboflavin as a redox mediator accelerating the reduction of the azo dye Mordant Yellow 10 by anaerobic granular sludge. Water Sci Technology, 48, 187–193.
von Canstein, H., Ogawa, J., Shimizu, S., & Lloyd, J. R. (2008). Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Applied and Environmental Microbiology, 74, 615–623.
Pearce, C. I., Christie, R., Boothman, C., Canstein, H. V., Guthrie, J. T., & Lloyd, J. R. (2006). Reactive azo dye reduction by Shewanella strain J18 143. Biotechnology and Bioengineering, 95, 692–703.
Hong, Y., Guo, J., Xu, Z., Xu, M., & Sun, G. (2007). Humic substances act as electron acceptor and redox mediator for microbial dissimilatory azoreduction by Shewanella decolorationis S12. Journal of Microbiology and Biotechnology, 17, 428–437.
Khalid, A., Arshad, M., & Crowley, D. E. (2008). Decolorization of azo dyes by Shewanella sp. under saline conditions. Applied Microbiology and Biotechnology, 79, 1053–1059.
Akhavan, O., & Ghaderi, E. (2010). Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano, 4, 5731–5736.
Carliell, C. M., Barclay, S. J., Shaw, C., Wheatley, A. D., & Buckley, C. A. (1998). The effect of salts used in textile dyeing on microbial decolourisation of a reactive azo dye. Environmental Technology, 19, 1133–1137.
Acknowledgments
This subject was supported by the National Natural Science foundation of China (No. 21077019).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 756 kb)
Rights and permissions
About this article
Cite this article
Zhang, HK., Lu, H., Wang, J. et al. Accelerating Effect of Bio-Reduced Graphene Oxide on Decolorization of Acid Red 18 by Shewanella algae . Appl Biochem Biotechnol 174, 602–611 (2014). https://doi.org/10.1007/s12010-014-1106-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1106-9