Abstract
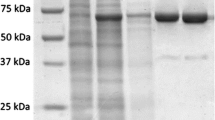
A family B DNA polymerase gene from the hyperthermophilic crenarchaeon Ignicoccus hospitalis KIN4/I was highly expressed under the control of T7lac promoter of pET-28ARG in Escherichia coli BL21-CodonPlus(DE3)-RIL cells. The produced I. hospitalis (Iho) DNA polymerase was purified by heat treatment followed by HisTrap™ HP column and HiTrap™ SP column chromatographies. The molecular mass of the purified Iho DNA polymerase was 88 kDa as estimated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The optimal pH for Iho DNA polymerase activity was 7.0 and the optimal temperature was 70 °C. Iho DNA polymerase was strongly activated by the presence of magnesium ion at an optimum concentration of 3 mM. The optimal concentration of KCl for Iho DNA polymerase activity was 60 mM. The half-life of the enzyme at 94 °C was about 2 h. The optimal conditions for polymerase chain reaction (PCR) were determined. Iho DNA polymerase possesses 3′ → 5′ exonuclease activity, and the fidelity of the Iho DNA polymerase was similar to that of Pfu and Vent DNA polymerases. However, Iho DNA polymerase provided more enhanced efficiency of PCR amplification than Pfu and Vent DNA polymerases. Iho DNA polymerase could successfully amplify a 2-kb λ DNA target with a 10-s extension time and could amplify a DNA fragment up to 8 kb λ DNA.





Similar content being viewed by others
References
Cann, I. K. O., Ishino, S., Nomura, N., Sako, Y., & Ishino, Y. (1999). Journal of Bacteriology, 181, 5984–5992.
Saiki, R. K., Gelfand, D. H., Stoffel, S., Scharf, S. J., Higuchi, R., Horn, G. T., Mullis, K. B., & Erlich, H. A. (1998). Science, 239, 487–491.
Lundberg, K. S., Shoemaker, D. D., Adams, M. W., Short, J. M., Sorge, J. A., & Mathur, E. J. (1991). Gene, 108, 1–6.
Kong, H., Kucera, R. B., & Jack, W. E. (1993). Journal of Biological Chemistry, 268, 1965–1975.
Takagi, M., Nishioka, M., Kakihara, H., Kitabayashi, M., Inoue, H., Kawakami, B., Oka, M., & Imanaka, T. (1997). Applied and Environmental Microbiology, 63, 4504–4510.
Griffiths, K., Nayak, S., Park, K., Mandelman, D., Modrell, B., Lee, J., Ng, B., Gibbs, M. D., & Bergquist, P. L. (2007). Protein Expression and Purification, 52, 19–30.
Marsic, D., Flaman, J. M., & Ng, J. D. (2008). Extremophiles, 12, 775–788.
Lee, J. I., Kim, Y. J., Bae, H. J., Cho, S. S., Lee, J. H., & Kwon, S. T. (2010). Applied Biochemistry and Biotechnology, 160, 1585–1599.
Gueguen, Y., Rolland, J. I., Lecompte, O., Azam, P., Romancer, G. L., Flament, D., Raffin, J. P., & Dietrich, J. (2001). European Journal of Biochemistry, 268, 5961–5969.
Uemori, T., Ishino, Y., Doi, H., & Kato, I. (1995). Journal of Bacteriology, 177, 2164–2177.
Ali, S. F., Rashid, N., Imanaka, T., & Akhtar, M. J. (2011). Bioscience and Bioengineering, 112, 118–123.
Edgell, D. R., Klenk, H. P., & Doolittle, W. F. (1997). Journal of Bacteriology, 179, 2632–2640.
Paper, W., Jahn, U., Hohn, M. J., Kronner, M., Näther, D. J., Durghardt, T., Rachel, R., Stetter, K. O., & Huber, H. (2007). International Journal of Systematic and Evolutionary Microbiology, 57, 803–808.
Podar, M., Anderson, I., Makarova, K. S., Elkins, J. G., Ivamova, N., Wall, M. A., Lykids, A., Mavoromatis, K., Sun, H., Hudson, M. E., Chen, W., Deciu, C., Hutchison, D., Eads, J. R., Anderson, A., Fernandes, F., Szeto, E., Lapidus, A., Kyrpides, N. C., Saier, M. H., Jr., Richardson, P. M., Rachel, R., Huber, H., Eisen, J. A., Koonin, E. V., Keller, M., & Stetter, K. O. (2008). Gemone Biology, 9, R158.1–18.
Choi, J. J., Nam, K. H., Min, B., Kim, S. J., Söll, D., & Kwon, S. T. (2006). Journal of Molecular Biology, 356, 1093–1106.
Kim, K. P., Cho, S. S., Lee, K. K., Youn, M. H., & Kwon, S. T. (2011). Journal of Biotechnology, 155, 156–163.
Cho, S. S., Kim, K. P., Lee, K. K., Yoon, M. H., & Kwon, S. T. (2012). Enzyme and Microbial Technology, 51, 334–341.
Choi, J. J., Song, J. G., Nam, K. H., Lee, J. I., Bae, H. J., Kim, G. A., Sun, Y., & Kwon, S. T. (2008). Applied and Environmental Microbiology, 74, 6563–6569.
Cline, J., Braman, J. C., & Hogrefe, H. H. (1996). Nucleic Acids Research, 24, 3546–3551.
Blanco, L., Bernad, A., Blasco, M. A., & Salas, M. (1991). Gene, 100, 27–38.
Ito, J., & Braithwaite, D. K. (1993). Nucleic Acids Research, 21, 787–802.
Wong, S. W., Wahl, A. F., Yuan, P. M., Aral, N., Pearson, B. E., Arai, K. I., Korn, D., Hunkapiller, M. W., & Wang, T. S. F. (1988). EMBO Journal, 7, 37–47.
Hamilton, S. C., Farchaus, J. W., & Davis, M. C. (2001). Biotechniques, 31, 370–383.
Zheng, R., Matsui, E., Shen, Y., Musti, K. V., Feng, Y., Darnis, S., Kawarabayasi, Y., Kikuchi, H., Harata, K., & Matsui, I. (2001). Extremophiles, 5, 111–117.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (NRF-2013R1A1A2008229).
Author information
Authors and Affiliations
Corresponding author
Additional information
Kang-Jin Seo and Sung Suk Cho have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Seo, KJ., Cho, S.S., Ppyun, H.W. et al. Characterization of a Family B DNA Polymerase from the Hyperthermophilic Crenarchaeon Ignicoccus hospitalis KIN4/I and Its Application to PCR. Appl Biochem Biotechnol 173, 1108–1120 (2014). https://doi.org/10.1007/s12010-014-0918-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0918-y