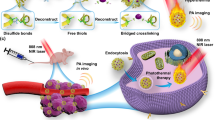
Abstract
Gold nanostructures have generated significant attention in biomedical areas because of their major role in cancer photothermal therapeutics. In order to conveniently combine gold nanostructures and drugs into one nanocomposite, Au2Se/Au core–shell nanostructures with strong near-infrared-absorbing properties were synthesized using a simple method and embedded inside bovine serum albumin (BSA) nanospheres by using a spray dryer equipped with an ultrasonic atomizer followed by thermal denaturation. The nanospheres with narrow size distribution mainly ranging from 450 to 600 nm were obtained. The Au2Se/Au-loaded BSA nanospheres (1 mg) adsorbed at least 0.01 mg of water-insoluble zinc phthalocyanine (ZnPc) photosensitizer. After irradiation with a 655-nm laser (20 min), the temperature of the Au2Se/Au-loaded BSA nanospheres [200 μL, 2 mg/mL, BSA/Au2Se/Au 10:1 (w/w)] increased by over 20 °C from the initial temperature of 24.82 ± 0.15 °C, and the release of ZnPc was improved compared with a corresponding sample without irradiation. After being incubated with cancer cells (human esophageal carcinoma Eca-109), the nanospheres exhibited photothermal and photodynamic therapy with a synergistic effect upon laser irradiation. This work provides novel Au2Se/Au-loaded polymer nanospheres prepared by a high-efficiency strategy for incorporating drugs for improving the efficiency in killing cancer cells.








Similar content being viewed by others
References
Raaphorst, G. P. (1990). Fundamental aspects of hyperthermic biology. In S. B. Field & J. W. Hand (Eds.), An introduction to the practical aspects of clinical hyperthermia (pp. 10–54). London: Taylor & Francis.
Fajardo, L. F. (1984). Pathological effects of hyperthermia in normal tissues. Cancer Research, 44, 4826s–4835s.
Hirsch, L. R., Stafford, R. J., Bankson, J. A., Sershen, S. R., Rivera, B., Price, R. E., et al. (2003). Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proceedings of the National Academy of Sciences of the United States of America, 100, 13549–13554.
Lowery, A. R., Gobin, A. M., Day, E. S., Halas, N. J., & West, J. L. (2006). Immunonanoshells for targeted photothermal ablation of tumor cell. International Journal of Nanomedicine, 1, 149–154.
Gobin, A. M., Lee, M. H., Halas, N. J., James, W. D., Drezek, R. A., & West, J. L. (2007). Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Letters, 7, 1929–1934.
Stern, J. M., Stanfield, J., Kabbani, W., Hsieh, J. T., & Cadeddu, J. A. (2008). Selective prostate cancer thermal ablation with laser activated gold nanoshells. Journal of Urology, 179, 748–753.
Park, H., Yang, J., Lee, J., Haam, S., Choi, I. H., & Yoo, K. H. (2009). Multifunctional nanoparticles for combined doxorubicin and photothermal treatments. ACS Nano, 3, 2919–2926.
Wu, C. Y., Yu, C., & Chu, M. Q. (2011). A gold nanoshell with a silica inner shell synthesized using liposome templates for doxorubicin loading and near-infrared photothermal therapy. International Journal of Nanomedicine, 6, 807–813.
Chen, J., Wang, D., Xi, J., Au, L., Siekkinen, A., Warsen, A., et al. (2007). Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Letters, 7, 1318–1322.
Skrabalak, S. E., Au, L., Lu, X., Li, X., & Xia, Y. (2007). Gold nanocages for cancer detection and treatment. Nanomedicine, 2, 657–668.
Hauck, T. S., Jennings, T. L., Yatsenko, T., Kumaradas, J. C., & Chan, W. C. W. (2008). Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Advanced Materials, 20, 3832–3838.
Huang, X. H., El-Sayed, I. H., Qian, W., & El-Sayed, M. A. (2006). Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. Journal of the American Chemical Society, 128, 2115–2120.
Wu, X., Ming, T., Wang, X., Wang, P. N., Wang, J. F., & Chen, J. Y. (2010). High-photoluminescence-yield gold nanocubes: for cell imaging and photothermal therapy. ACS Nano, 4, 113–120.
Liu, X. W., Tao, H. Q., Yang, K., Zhang, S. A., Lee, S. T., & Liu, Z. A. (2011). Optimization of surface chemistry on single-walled carbon nanotubes for in vivo photothermal ablation of tumors. Biomaterials, 32, 144–151.
Markovic, Z. M., Harhaji-Trajkovic, L. M., Todorovic-Markovic, B. M., Kepic, D. P., Arsikin, K. M., Jovanovic, S. P., et al. (2011). In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials, 32, 1121–1129.
Huang, N. Y., Wang, H. Q., Zhao, J. H., Lui, H., Korbelik, M., & Zeng, H. S. (2010). Single-wall carbon nanotubes assisted photothermal cancer therapy: animal study with a murine model of squamous cell carcinoma. Lasers in Surgery and Medicine, 42, 638–648.
Burke, A., Ding, X. F., Singh, R., Kraft, R. A., Levi-Polyachenko, N., Rylander, M. N., et al. (2009). Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proceedings of the National Academy of Sciences of the United States of America, 106, 12897–12902.
Chakravarty, P., Marches, R., Zimmerman, N. S., Swafford, A. D., Bajaj, P., Musselman, I. H., et al. (2008). Thermal ablation of tumor cells with antibody-functionalized single-walled carbon nanotubes. Proceedings of the National Academy of Sciences of the United States of America, 105, 8697–8702.
Zhang, M. F., Murakami, T., Ajima, K., Tsuchida, K., Sandanayaka, A. S., Ito, O., et al. (2008). Fabrication of ZnPc/protein nanohorns for double photodynamic and hyperthermic cancer phototherapy. Proceedings of the National Academy of Sciences of the United States of America, 105, 14773–14778.
Yang, K., Zhang, S., Zhang, G., Sun, X., Lee, S. T., & Liu, Z. (2010). Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Letters, 10, 3318–3323.
Chu, M., Peng, P., Zhao, J., Liang, S., Shao, Y., & Wu, Q. (2013). Laser light triggered-activated carbon nanosystem for cancer therapy. Biomaterials, 34, 1820–1832.
Chu, M., Pan, X., Zhang, D., Wu, Q., Peng, J., & Hai, W. (2012). The therapeutic efficacy of CdTe and CdSe quantum dots for photothermal cancer therapy. Biomaterials, 33, 7071–7083.
Abels, C., Karrer, S., BaumLer, W., Goetz, A. E., Landthaler, M., & Szeimies, R. M. (1998). Indocyanine green and laser light for the treatment of AIDS-associated cutaneous Kaposi's sarcoma. British Journal of Cancer, 77, 1021–1024.
Zheng, X., Xing, D., Zhou, F., Wu, B., & Chen, W. R. (2011). Indocyanine green-containing nanostructure as near infrared dual-functional targeting probes for optical imaging and photothermal therapy. Molecular Pharmaceutics, 8, 447–456.
Lim, H. J., & Oh, C. H. (2011). Indocyanine green-based photodynamic therapy with 785 nm light emitting diode for oral squamous cancer cells. Photodiagnosis and Photodynamic, 8, 337–342.
Huang, X. H., Jain, P. K., El-Sayed, I. H., & El-Sayed, M. A. (2008). Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers in Medical Science, 23, 217–228.
Huang, X. H., & El-Sayed, M. A. (2010). Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. Journal of Advanced Research, 1, 13–28.
Jelveh, S., & Chithrani, D. B. (2011). Gold nanostructures as a platform for combinational therapy in future cancer therapeutics. Cancers, 3, 1081–1110.
Day, E. S., Bickford, L. R., Slater, J. H., Riggall, N. S., Drezek, R. A., & West, J. L. (2010). Antibody-conjugated gold-gold sulfide nanoparticles as multifunctional agents for imaging and therapy of breast cancer. International Journal of Nanomedicine, 5, 445–454.
Gobin, A. M., Watkins, E. M., Quevedo, E., Colvin, V. L., & West, J. L. (2010). Near-infrared-resonant gold/gold sulfide nanoparticles as a photothermal cancer therapeutic agent. Small, 6, 745–752.
Jin, H., Hong, B., Kakar, S. S., & Kang, K. A. (2008). Tumor-specific nano-entities for optical detection and hyperthermic treatment of breast cancer. Advances in Experimental Medicine and Biology, 614, 275–284.
Paddock, C. (2010). Gold-coated liposomes could make chemo more effective, less harmful. Available from: www.mednewstoday.com. Accessed October 1, 2011.
Jang, B., Park, J. Y., Tung, C. H., Kim, I. H., & Choi, Y. (2011). Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano, 5, 1086–1094.
You, J., Zhang, G. D., & Li, C. (2010). Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano, 4, 1033–1041.
Yavuz, M. S., Cheng, Y. Y., Chen, J. Y., Cobley, C. M., Zhang, Q., Rycenga, M., et al. (2009). Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nature Materials, 8, 935–939.
Norman, T. J., Grant, C. D., Magana, D., Zhang, J. Z., Liu, J., Cao, D. L., et al. (2002). Near infrared optical absorption of gold nanoparticle aggregates. The Journal of Physical Chemistry. B, 106, 7005–7012.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (81071833), the Natural Science Foundation of Jiangsu Province (SBK201123093), and the Program for New Century Excellent Talents in University (NCET-07-0618).
Author information
Authors and Affiliations
Corresponding author
Additional information
Cong Yu, Fangjie Wo, and Yuxiang Shao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yu, C., Wo, F., Shao, Y. et al. Bovine Serum Albumin Nanospheres Synchronously Encapsulating “Gold Selenium/Gold” Nanoparticles and Photosensitizer for High-Efficiency Cancer Phototherapy. Appl Biochem Biotechnol 169, 1566–1578 (2013). https://doi.org/10.1007/s12010-012-0078-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-0078-x