Abstract
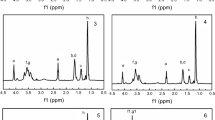
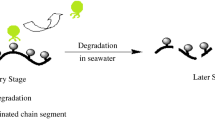
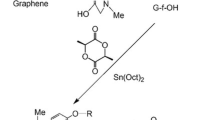
Poly(ether ester) polyols consisting of polypropylene glycol-2000 (PPG-2000) and different ratios of ε-caprolactone and lactic acid (LA) had been prepared by polycondensation reactions. Degradable polyurethane (PU) films were prepared with poly (ether ester) polyols and hexamethylene diisocyanate trimer. Hydrolytic degradation experiments demonstrated that PU films could be degradable in artificial seawater. Importantly, the hydrolytic degradation of PU films in artificial seawater increased with the increase of LA content. The results also showed that the surfaces of PU films were renewed and kept active, as revealed by the scanning electron microscope micrographs of degradation PU coatings. Moreover, the copper ion release rates of PU coatings prepared with poly(ether ester) polyols reached steady state at about 35 days. The degradable polyurethanes based on poly(ether ester) polyols (PPG-2000, ε-caprolactone, and LA) could be effective and durable resins for marine antifouling.







Similar content being viewed by others
References
Ma, C, Xu, L, Xua, W, Zhang, G, “Degradable Polyurethane for Marine Antifouling.” J. Mater. Chem. B, 1 3099–3106 (2013)
Meseguer Yebra, D, Kiil, S, Dam-Johansen, K, “Antifouling Technology—Past, Present and Future Steps Towards Efficient and Environmentally Friendly Antifouling Coatings.” Prog. Org. Coat., 50 75–104 (2004)
Yonehara, Y, Yamashita, H, Kawamura, C, Itoh, K, “A New Antifouling Coating Based on a Zinc Acrylate Copolymer.” Prog. Org. Coat., 42 150–158 (2001)
Rascio, VJD, Giúdice, CA, del Ami, B, “Research and Development of Soluble Matrix Antifouling Paints for Ships, Offshore Platforms and Power Stations. A Review.” Corros. Rev., 8 87–153 (1988)
CEPE Antifouling Working Group, Final Report, EC Project., 96/559/3040/DEB/E2 (1999)
Swain, G, Proceedings of the International Symposium on Sea water Drag Reduction. The Naval Undersea Warfare Center, Newport, pp. 155–161 (1998)
Evans, SM, Leksono, T, McKinnel, PD, “Tributyltin Pollution: A Diminishing Problem Following Legislation Limiting the Use of TBT-Based Anti-fouling Paints.” Mar. Pollut. Bull., 30 (1) 14–21 (1995)
Omae, I, “General Aspects of Tin-Free Antifouling Paints.” Chem. Rev., 103 3431–3448 (2003)
Voulvoulis, N, Scrimshaw, MD, Lester, JN, “Alternative Antifouling Biocides.” Appl. Organomet. Chem., 13 135 (1999)
Lewis, AG, Copper in Water Aquatic Environments. International Copper Association, New York, 1994
Lewis, AG, Cave, WR, “The Biological Importance of Copper in Oceans and Estuaries.” Oceanogr. Mar. Biol. Annu. Rev., 20 471 (1982)
Vasconcelos, MTSD, Leal, MFC, "Adsorption and Uptake of Cu by Emiliania huxleyi in Natural Seawater." Environ. Sci. Technol., 35 508–515 (2001)
Fay, F, Linossier, I, Jacques Peron, J, Langlois, V, Vallee-Rehel, K, “Antifouling Activity of Marine Coatings: Study of Erosion.” Prog. Org. Coat., 60 194–206 (2007)
Hugues, C, Bressy, C, Bartolomeo, P, Margaillan, A, “Complexation of an Acrylic Resin by Tertiary Amines: Synthesis and Characterisation of New Binders for Antifouling Coatings.” Eur. Polym. J., 39 319–326 (2003)
Báez, JE, Marcos-Fernández, Á, “Degradable Poly(ester-ether urethane)s Derived of AB2miktoarm Star Copolymer Poly(ethylene glycol-(e-caprolactone)2) Diol: Synthesis, Characterization and Degradation.” React. Funct. Polym., 72 349–357 (2010)
Fay, F, Linossier, I, Langlois, V, Renard, E, Vallee-Rehel, K, “Degradation and Controlled Release Behavior of ε-Caprolactone Copolymers in Degradable Antifouling Coatings.” Biomacromolecules, 7 851–857 (2006)
Fay, F, Renard, E, Langlois, V, Linossier, I, Vallee-Rehel, K, “Development of Poly(ε-caprolactone-co-l-lactide) and Poly(ε-caprolactone-co-d-valerolactone) as New Degradable Binder Used for Antifouling Coating.” Eur. Polym. J., 43 4800–4813 (2007)
Bressy, C, Margaillan, A, “Erosion Study of Poly(trialkylsilyl methacrylate)-Based Antifouling Coatings.” Prog. Org. Coat., 66 400–405 (2009)
Liu, C, Qian, Z, Gu, Y, “Synthesis, Characterization, and Thermal Properties of Degradable Aliphatic Copolyester Based on q-Caprolactone, Adipic Acid, and 1,6-Hexanediol.” Mater. Lett., 60 31–38 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yi, J., Ren, R., Huang, C. et al. Synthesis and characterization of degradable polyurethane based on poly(ether ester) polyols (PPG-2000 and ε-caprolactone/lactic acid) for marine antifouling. J Coat Technol Res 12, 525–532 (2015). https://doi.org/10.1007/s11998-014-9646-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-014-9646-z