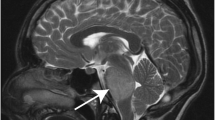
Abstract
Purpose of review
This review of diffuse intrinsic pontine glioma (DIPG) provides clinical background, a systematic approach to diagnosis and initial care, and synthesizes historical, modern, and future directions for treatment. We present evidence supporting neurosurgical biopsy, early palliative care involvement, limitation of glucocorticoid use, and the leveraging of preclinical DIPG models as a pipeline to next-generation clinical trials.
Recent findings
New molecular understanding of pediatric high-grade gliomas has led to the reclassification of DIPG as one member of a family of diffuse gliomas occurring in the midline of the central nervous system that exhibit pathognomonic mutations in genes encoding histone 3 (H3 K27M). DIPG remains a clinically relevant term, though diagnostically the 80% of DIPG cases that exhibit the H3 K27M mutation have been reclassified as diffuse midline glioma, H3 K27M-mutant. Re-irradiation has been shown to be well-tolerated and of potential benefit. Epigenetic targeting of transcriptional dependencies in preclinical models is fueling molecularly targeted clinical trials. Chimeric antigen receptor T cell immunotherapy has also demonstrated efficacy in preclinical models and provides a promising new clinical strategy.
Summary
DIPG is a universally fatal, epigenetically driven tumor of the pons that is considered part of a broader class of diffuse midline gliomas sharing H3 K27M mutations. Radiation remains the standard of care, single-agent temozolomide is not recommended, and glucocorticoids should be used only sparingly. A rapid evolution of understanding in the chromatin, signaling, and immunological biology of DIPG may soon result in clinical breakthroughs.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Harris W. A case of pontine glioma, with special reference to the paths of gustatory sensation. Proc R Soc Med. 1926;19(Neurol Sect):1–5.
Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–20.
•• Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–47.
•• Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–3.
•• Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodeling genes in pediatric glioblastoma. Nature. 2012;482(7384):226–31.Wu et al., Khuong-Quang et al. and Schwartzentruber et al. discovered the highly recurrent H3 K27M mutation in DIPG and other pediatric midline gliomas. This discovery of an “oncohistone” has revolutionized our understanding of the pathophysiology of this disease and underscores the central role for epigenetic dysregulation in DIPG and other pediatric malignancies.
Albright AL, Packer RJ, Zimmerman R, et al. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children’s Cancer Group. Neurosurgery. 1993;33(6):1026–9 discussion 1029–30.
Hankinson TC, Campagna EJ, Foreman NK, et al. Interpretation of magnetic resonance images in diffuse intrinsic pontine glioma: a survey of pediatric neurosurgeons. J Neurosurg Pediatr. 2011;8(1):97–102.
Barkovich AJ, Krischer J, Kun LE, et al. Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990;16(2):73–83.
Pajtler, K.W., S.C. Mack, V. Ramaswamy, et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2016.
Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–72.
Reis GF, Tihan T. Therapeutic targets in pilocytic astrocytoma based on genetic analysis. Semin Pediatr Neurol. 2015;22(1):23–7.
Lapin DH, Tsoli M, Ziegler DS. Genomic insights into diffuse intrinsic pontine glioma. Front Oncol. 2017;7:57.
• Lieberman NAP, Vitanza NA, Crane CA. Immunotherapy for brain tumors: understanding early successes and limitations. Expert Rev Neurother. 2018;18(3):251–9.
Lieberman NAP, DeGolier K, Kovar HM, et al. Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: implications for development of immunotherapy. Neuro Oncol. 2019;21(1):83–94.
•• Nagaraja S, Vitanza NA, Woo PJ, et al. Transcriptional dependencies in diffuse intrinsic pontine glioma. Cancer Cell. 2017, 31(5):635–652 e6.This preclinical work discovered DIPG’s vulnerabilities to BRD4 and CDK7 blockade, as well as their synergistic benefit when combined with HDAC inhibition.
• Lin GL, Nagaraja S, Filbin MG, et al. Non-inflammatory tumor microenvironment of diffuse intrinsic pontine glioma. Acta Neuropathol Commun. 2018;6(1):51 Lin et al. and Lieberman et al. independently discovered the microenvironment in DIPG is neither immunosuppresive nor inflammatory, making it distinct from that of adult GBM.
Becher OJ, Hambardzumyan D, Walker TR, et al. Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer Res. 2010;70(6):2548–57.
Biery, M., C. Myers, E. Girard, et al. A novel HDAC inhibitor in new patient-derived diffuse intrinsic pontine glioma (DIPG) models, in ISPNO2018 - International Symposium on Pediatric Neuro-Oncology. DIPG-35, Presentation. 2018: Denver, CO, USA. p. i56.
Cage TA, Samagh SP, Mueller S, et al. Feasibility, safety, and indications for surgical biopsy of intrinsic brainstem tumors in children. Childs Nerv Syst. 2013;29(8):1313–9.
Grasso CS, Tang Y, Truffaux N, et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. 2015;21(6):555–9.
• Gupta, N., L.C. Goumnerova, P. Manley, et al. Prospective feasibility and safety assessment of surgical biopsy for patients with newly diagnosed diffuse intrinsic pontine glioma. Neuro Oncol. 2018;20(11):1547–1555.An important prospective study evaluating the safety of biopsy for patients with DIPG.
Larson JD, Kasper LH, Paugh BS, et al. Histone H3.3 K27M accelerates spontaneous brainstem glioma and drives restricted changes in bivalent gene expression. Cancer Cell. 2019;35(1):140–155 e7.
Lin GL, Monje M. A protocol for rapid post-mortem cell culture of diffuse intrinsic pontine glioma (DIPG). J Vis Exp. 2017;(121).
Puget S, Beccaria K, Blauwblomme T, et al. Biopsy in a series of 130 pediatric diffuse intrinsic pontine gliomas. Childs Nerv Syst. 2015;31(10):1773–80.
Roujeau T, Machado G, Garnett MR, et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107(1 Suppl):1–4.
Tsoli M, Shen H, Mayoh C, et al. International experience in the development of patient-derived xenograft models of diffuse intrinsic pontine glioma. J Neurooncol. 2018.
Wang ZJ, Rao L, Bhambhani K, et al. Diffuse intrinsic pontine glioma biopsy: a single institution experience. Pediatr Blood Cancer. 2015;62(1):163–5.
Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys. 1998;40(2):265–71.
• Cooney T, Lane A, Bartels U, et al. Contemporary survival endpoints: an International Diffuse Intrinsic Pontine Glioma Registry study. Neuro Oncol. 2017;19(9):1279–80 An important update on international survival endpoints for children with DIPG.
Veldhuijzen van Zanten SE, Jansen MH, Sanchez Aliaga E, et al. A twenty-year review of diagnosing and treating children with diffuse intrinsic pontine glioma in The Netherlands. Expert Rev Anticancer Ther. 2014;15(2):157–64
Lassman LP, Arjona VE. Pontine gliomas of childhood. Lancet. 1967;1(7496):913–5.
Fisher PG, Breiter SN, Carson BS, et al. A clinicopathologic reappraisal of brain stem tumor classification. Identification of pilocystic astrocytoma and fibrillary astrocytoma as distinct entities. Cancer. 2000;89(7):1569–76.
Fried I, Hawkins C, Scheinemann K, et al. Favorable outcome with conservative treatment for children with low grade brainstem tumors. Pediatr Blood Cancer. 2012;58(4):556–60.
Giussani C, Poliakov A, Ferri RT, et al. DTI fiber tracking to differentiate demyelinating diseases from diffuse brain stem glioma. Neuroimage. 2010;52(1):217–23.
Lober RM, Cho YJ, Tang Y, et al. Diffusion-weighted MRI derived apparent diffusion coefficient identifies prognostically distinct subgroups of pediatric diffuse intrinsic pontine glioma. J Neurooncol. 2014;117(1):175–82.
Caretti V, Bugiani M, Freret M, et al. Subventricular spread of diffuse intrinsic pontine glioma. Acta Neuropathol. 2014;128(4):605–7.
• Huang TY, Piunti A, Lulla RR, et al. Detection of Histone H3 mutations in cerebrospinal fluid-derived tumor DNA from children with diffuse midline glioma. Acta Neuropathol Commun. 2017;5(1):28.Considering DIPG biopsy’s requirement of neurosurgical precision, limited availability, low but significant risk of complications, and the decision’s emotional toll on families, this is an important study showing histone H3 mutations can be detected in CSF.
Saratsis AM, Yadavilli S, Magge S, et al. Insights into pediatric diffuse intrinsic pontine glioma through proteomic analysis of cerebrospinal fluid. Neuro Oncol. 2012;14(5):547–60.
Pan C, Diplas BH, Chen X, et al. Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol. 2018;137(2):297–306.
Buczkowicz P, Bartels U, Bouffet E, et al. Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol. 2014;128(4):573–81.
Bozkurt SU, Dagcinar A, Tanrikulu B, et al. Significance of H3K27M mutation with specific histomorphological features and associated molecular alterations in pediatric high-grade glial tumors. Childs Nerv Syst. 2018;34(1):107–16.
Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. J Mol Diagn. 2014;16(1):56–67.
Lewis PW, Muller MM, Koletsky MS, et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340(6134):857–61.
Shankar GM, Lelic N, Gill CM, et al. BRAF alteration status and the histone H3F3A gene K27M mutation segregate spinal cord astrocytoma histology. Acta Neuropathol. 2016;131(1):147–50.
•• Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32(4):520–537. A comprehensive analysis of DIPG’s molecular aberrations and their clinical significance.
Guida L, Roux FE, Massimino M, et al. Safety and efficacy of endoscopic third ventriculostomy in diffuse intrinsic pontine glioma related hydrocephalus: a systematic review. World Neurosurg. 2019;124:29–35.
Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13.
Drozdowicz LB, Bostwick JM. Psychiatric adverse effects of pediatric corticosteroid use. Mayo Clin Proc. 2014;89(6):817–34.
Goforth P, Gudas CJ. Effects of steroids on wound healing: a review of the literature. J Foot Surg. 1980;19(1):22–8.
Pappachan JM, Hariman C, Edavalath M, et al. Cushing’s syndrome: a practical approach to diagnosis and differential diagnoses. J Clin Pathol. 2017;70(4):350–9.
Fauquette W, Amourette C, Dehouck MP, et al. Radiation-induced blood-brain barrier damages: an in vitro study. Brain Res. 2012;1433:114–26.
Hue CD, Cho FS, Cao S, et al. Dexamethasone potentiates in vitro blood-brain barrier recovery after primary blast injury by glucocorticoid receptor-mediated upregulation of ZO-1 tight junction protein. J Cereb Blood Flow Metab. 2015;35(7):1191–8.
• Luedi MM, Singh SK, Mosley JC, et al. A dexamethasone-regulated gene signature is prognostic for poor survival in glioblastoma patients. J Neurosurg Anesthesiol. 2017;29(1):46–58.This work highlights the importance of limiting steroid use in our patients.
Pitter KL, Tamagno I, Alikhanyan K, et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(Pt 5):1458–71.
Mandrell BN, Baker J, Levine D, et al. Children with minimal chance for cure: parent proxy of the child’s health-related quality of life and the effect on parental physical and mental health during treatment. J Neurooncol. 2016;129(2):373–81.
Langmoen IA, Lundar T, Storm-Mathisen I, et al. Management of pediatric pontine gliomas. Childs Nerv Syst. 1991;7(1):13–5.
Janssens GO, Jansen MH, Lauwers SJ, et al. Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: a matched-cohort analysis. Int J Radiat Oncol Biol Phys. 2013;85(2):315–20.
Zaghloul MS, Eldebawy E, Ahmed S, et al. Hypofractionated conformal radiotherapy for pediatric diffuse intrinsic pontine glioma (DIPG): a randomized controlled trial. Radiother Oncol. 2014;111(1):35–40.
Hankinson TC, Patibandla MR, Green A, et al. Hypofractionated radiotherapy for children with diffuse intrinsic pontine gliomas. Pediatr Blood Cancer. 2015.
Packer RJ, Boyett JM, Zimmerman RA, et al. Outcome of children with brain stem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Childrens Cancer Group Phase I/II Trial. Cancer. 1994;74(6):1827–34.
Freese C, Takiar V, Fouladi M, et al. Radiation and subsequent reirradiation outcomes in the treatment of diffuse intrinsic pontine glioma and a systematic review of the reirradiation literature. Pract Radiat Oncol. 2017;7(2):86–92.
Janssens GO, Gandola L, Bolle S, et al. Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: a matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur J Cancer. 2017;73:38–47.
Lassaletta A, Strother D, Laperriere N, et al. Reirradiation in patients with diffuse intrinsic pontine gliomas: the Canadian experience. Pediatr Blood Cancer. 2018;65(6):e26988.
Morales La Madrid A, Santa-Maria V, Cruz Martinez O, et al. Second re-irradiation for DIPG progression, re-considering “old strategies” with new approaches. Childs Nerv Syst. 2017;33(5):849–52.
Robison NJ, Kieran MW. Diffuse intrinsic pontine glioma: a reassessment. J Neurooncol. 2014;119(1):7–15.
Aquino-Parsons C, Hukin J, Green A. Concurrent carbogen and radiation therapy in children with high-risk brainstem gliomas. Pediatr Blood Cancer. 2008;50(2):397–9.
Bradley KA, Zhou T, McNall-Knapp RY, et al. Motexafin-gadolinium and involved field radiation therapy for intrinsic pontine glioma of childhood: a children’s oncology group phase 2 study. Int J Radiat Oncol Biol Phys. 2013;85(1):e55–60.
Freeman CR, Kepner J, Kun LE, et al. A detrimental effect of a combined chemotherapy-radiotherapy approach in children with diffuse intrinsic brain stem gliomas? Int J Radiat Oncol Biol Phys. 2000;47(3):561–4.
Massimino M, Spreafico F, Biassoni V, et al. Diffuse pontine gliomas in children: changing strategies, changing results? A mono-institutional 20-year experience. J Neurooncol. 2008;87(3):355–61.
Wagner S, Warmuth-Metz M, Emser A, et al. Treatment options in childhood pontine gliomas. J Neurooncol. 2006;79(3):281–7.
Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol. 2011;13(4):410–6.
Jalali R, Raut N, Arora B, et al. Prospective evaluation of radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys. 2010;77(1):113–8.
Sharp JR, Bouffet E, Stempak D, et al. A multi-centre Canadian pilot study of metronomic temozolomide combined with radiotherapy for newly diagnosed paediatric brainstem glioma. Eur J Cancer. 2010;46(18):3271–9.
Bailey S, Howman A, Wheatley K, et al. Diffuse intrinsic pontine glioma treated with prolonged temozolomide and radiotherapy--results of a United Kingdom phase II trial (CNS 2007 04). Eur J Cancer. 2013;49(18):3856–62.
Broniscer A, Baker JN, Tagen M, et al. Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma. J Clin Oncol. 2010;28(31):4762–8.
Broniscer A, Baker SD, Wetmore C, et al. Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma. Clin Cancer Res. 2013;19(11):3050–8.
Geoerger B, Hargrave D, Thomas F, et al. Innovative therapies for children with cancer pediatric phase I study of erlotinib in brainstem glioma and relapsing/refractory brain tumors. Neuro Oncol. 2011;13(1):109–18.
Haas-Kogan DA, Banerjee A, Poussaint TY, et al. Phase II trial of tipifarnib and radiation in children with newly diagnosed diffuse intrinsic pontine gliomas. Neuro Oncol. 2011;13(3):298–306.
Pollack IF, Jakacki RI, Blaney SM, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric Brain Tumor Consortium report. Neuro Oncol. 2007;9(2):145–60.
Bartels U, Wolff J, Gore L, et al. Phase 2 study of safety and efficacy of nimotuzumab in pediatric patients with progressive diffuse intrinsic pontine glioma. Neuro Oncol. 2014;16(11):1554–9.
Veldhuijzen van Zanten SEM, El-Khouly FE, Jansen MHA, et al. A phase I/II study of gemcitabine during radiotherapy in children with newly diagnosed diffuse intrinsic pontine glioma. J Neurooncol. 2017;135(2):307–15.
Kilburn LB, Kocak M, Baxter P, et al. A pediatric brain tumor consortium phase II trial of capecitabine rapidly disintegrating tablets with concomitant radiation therapy in children with newly diagnosed diffuse intrinsic pontine gliomas. Pediatr Blood Cancer. 2018;65(2):e26832.
Pollack IF, Stewart CF, Kocak M, et al. A phase II study of gefitinib and irradiation in children with newly diagnosed brainstem gliomas: a report from the Pediatric Brain Tumor Consortium. Neuro Oncol. 2011;13(3):290–7.
Hashizume R, Andor N, Ihara Y, et al. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat Med. 2014;20(12):1394–6.
• Vitanza NA, Johnson A, Beebe A, et al. Locoregional HER2CAR T cells for pediatric central nervous system tumors: preclinical efficacy to tolerability in first patient. IMMU-02, Oral Presentation, in Society of Neuro-Oncology Pediatric Basic and Translational Research Conference. 2019: San Francisco, CA. This work highlights the initial patient experience in locoregionally delivering HER2 CAR T cells to children with recurrent/refractory CNS tumors, providing a framework for future locoregional DIPG CAR T cell trials.
Ahmed N, Brawley V, Hegde M, et al. HER2-Specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3(8):1094–101.
Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–31.
• Mount CW, Majzner RG, Sundaresh S, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat Med. 2018;24(5):572–9.The first published DIPG-specific preclinical CAR T cell work, highlighting the vulnerability of targeting GD2.
• Majzner RG, Theruvath JL, Nellan A, et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. 2019;25(8):2560–2574.B7-H3 has been identified as a surface antigen present in many pediatric CNS tumors and this preclinical work served as the foundation for upcoming B7-H3 CAR T cell trials for pediatric CNS tumors including DIPG.
Halle B, Mongelard K, Poulsen FR. Convection-enhanced drug delivery for glioblastoma: a systematic review focused on methodological differences in the use of the convection-enhanced delivery method. Asian J Neurosurg. 2019;14(1):5–14.
Souweidane MM, Kramer K, Pandit-Taskar N, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018;19(8):1040–50.
Acknowledgements
The authors gratefully acknowledge support from the National Institute of Neurological Disorders and Stroke (R01NS092597), NIH Director’s Pioneer Award (DP1NS111132), Unravel Pediatric Cancer, McKenna Claire Foundation, Virginia and D.K. Ludwig Fund for Cancer Research, ChadTough Foundation, Defeat DIPG, and Abbie’s Army Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Nicholas A. Vitanza declares no potential conflicts of interest. Michelle Monje has a pending patent entitled “CAR T cell therapy to treat H3K27M midline gliomas.”
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Vitanza, N.A., Monje, M. Diffuse Intrinsic Pontine Glioma: From Diagnosis to Next-Generation Clinical Trials. Curr Treat Options Neurol 21, 37 (2019). https://doi.org/10.1007/s11940-019-0577-y
Published:
DOI: https://doi.org/10.1007/s11940-019-0577-y