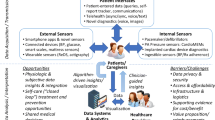
Abstract
Purpose of Review
Heart failure is an important clinical and public health issue. There is an urgent need to improve the efficiency of clinical trials in heart failure to rapidly identify new therapies and evidence-based implementation strategies for currently existing therapies. Electronic health (eHealth) platforms and digital health tools are being integrated into heart failure care. In this manuscript, we review opportunities to use these tools to potentially improve the design of and reduce the complexity of clinical trials in heart failure.
Recent Findings
The PRECIS-2 tool outlines clinical trial design domains that are targets for pragmatism. We believe incorporating pragmatic design elements with the aid of eHealth platforms and digital health tools into clinical trials may help address the current complexity of clinical trials in heart failure and improve efficiency. In the manuscript, we provide examples from recent clinical trials across clinical trial design domains.
Summary
We believe the current adoption of eHealth platforms and digital health tools is an opportunity improve the design of heart failure clinical trials. We specifically believe these tools can enhance pragmatism in clinical trials and reduce delays in generating high-quality evidence for new heart failure therapeutics.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Roger VL. Epidemiology of heart failure. Circ Res. 2021;128(10):1421–34.
Savarese G, et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118(17):3272–87.
Lund LH, Oldgren J, James S. Registry-based pragmatic trials in heart failure: current experience and future directions. Curr Heart Fail Rep. 2017;14(2):59–70.
O’Connor CM, et al. Improving heart failure therapeutics development in the United States: the heart failure collaboratory. J Am Coll Cardiol. 2018;71(4):443–53.
• Haywood HB, et al. The Promise and Risks of mHealth in Heart Failure Care. J Card Fail. 2023;29(9):1298–310. https://doi.org/10.1016/j.cardfail.2023.07.005. This contemporary review provides additional information on the use of eHealth platforms and digital health tools in heart failure care.
Allen LA, et al. Effectiveness of an intervention supporting shared decision making for destination therapy left ventricular assist device: the DECIDE-LVAD randomized clinical trial. JAMA Intern Med. 2018;178(4):520–9.
Califf RM, Sugarman J. Exploring the ethical and regulatory issues in pragmatic clinical trials. Clin Trials. 2015;12(5):436–41.
Mentz RJ, et al. Effect of torsemide vs furosemide after discharge on all-cause mortality in patients hospitalized with heart failure: the TRANSFORM-HF randomized clinical trial. JAMA. 2023;329(3):214–23.
Stone GW, et al. Five-year follow-up after transcatheter repair of secondary mitral regurgitation. N Engl J Med. 2023;388(22):2037–48.
• Loudon K, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. The Pragmatic Explanatory Continuum Indicator Summary 2 is an established tool to aid investigators in understanding where specific exist on a continuum between explanatory and pragmatic trials.
Solomon SD, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–98.
Peters AE, et al. A multicenter program for electronic health record screening for patients with heart failure with preserved ejection fraction: lessons from the DELIVER-EHR initiative. Contemp Clin Trials. 2022;121:106924.
Reading Turchioe M, et al. Systematic review of current natural language processing methods and applications in cardiology. Heart. 2022;108(12):909–16.
Jonnalagadda SR, et al. Text mining of the electronic health record: an information extraction approach for automated identification and subphenotyping of HFpEF patients for clinical trials. J Cardiovasc Transl Res. 2017;10(3):313–21.
Allen LA, et al. An electronically delivered patient-activation tool for intensification of medications for chronic heart failure with reduced ejection fraction. Circulation. 2021;143(5):427–37.
Ghazi L, et al. Electronic alerts to improve heart failure therapy in outpatient practice. J Am Coll Cardiol. 2022;79(22):2203–13.
Aktaa S, et al. Data standards for heart failure: the European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart). Eur Heart J. 2022;43(23):2185–95.
Perez MV, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909–17.
Nassif M, et al. Recruitment strategies of a decentralized randomized placebo controlled clinical trial: the canagliflozin impact on health status, quality of life and functional status in heart failure (CHIEF-HF) trial. J Card Fail. 2023;29(6):863–9. https://doi.org/10.1016/j.cardfail.2023.04.001.
Spertus JA, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. 2022;28(4):809–13.
Heidenreich PA, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032.
Ruppar TM, et al. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials. J Am Heart Assoc. 2016 ;5(6):e002606. https://doi.org/10.1161/JAHA.115.002606.
Felker GM, et al. A randomized controlled trial of mobile health intervention in patients with heart failure and diabetes. J Cardiac Fail. 2022;28(11):1575–83.
Kotecha D, et al. CODE-EHR best practice framework for the use of structured electronic healthcare records in clinical research. Eur Heart J. 2022;43(37):3578–88.
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by the first author and all authors critically reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The following relationships exist related to this manuscript:
Adam DeVore reports research funding through his institution from Biofourmis, Bodyport, Cytokinetics, American Regent, Inc, the NIH and NHLBI, Novartis, and Story Health. He also provides consulting services for and/or receives honoraria from Abiomed, Cardionomic, LivaNova, Natera, NovoNordisk, Story Health, and Zoll.
Marat Fudim received consulting fees from Abbott, Ajax, Alio Health, Alleviant, Artha, Audicor, AxonTherapies, Bayer, Bodyguide, Bodyport, Boston Scientific, Broadview, Cadence, Cardioflow, Cardionomics, Coridea, CVRx, Daxor, Deerfield Catalyst, Edwards LifeSciences, Echosens, EKO, Feldschuh Foundation, Fire1, FutureCardia, Galvani, Gradient, Hatteras, HemodynamiQ, Impulse Dynamics, Intershunt, Medtronic, Merck, NIMedical, NovoNordisk, NucleusRx, NXT Biomedical, Orchestra, Pharmacosmos, PreHealth, Presidio, Procyreon, ReCor, Rockley, SCPharma, Shifamed, Splendo, Summacor, SyMap, Verily, Vironix, Viscardia, Zoll.
Lars Lund reports: Grants, consulting, honoraria: Abbot, Alleviant, AstraZeneca, Bayer, Biopeutics, Boehringer Ingelheim, Edwards, Merck/MSD, Novartis, Novo Nordisk, OrionPharma, Owkin, Pharmacosmos, Vifor Pharma; Stock ownership: AnaCardio.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
DeVore, A.D., Fudim, M. & Lund, L.H. Novel Trial Designs in Heart Failure: Using Digital Health Tools to Increase Pragmatism. Curr Heart Fail Rep 21, 5–10 (2024). https://doi.org/10.1007/s11897-023-00640-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-023-00640-y