Abstract
Conventional therapies remain the mainstay of treatment for most patients with inflammatory bowel disease (IBD), with only a minority of patients requiring biologic therapies. Recently, attention has focused on optimizing dosing strategies for biologic agents; however, of equal importance are recent advances in the optimization of conventional IBD therapies. Newer aminosalicylate formulations demonstrate similar efficacy with a reduced pill burden and less frequent dosing, while new corticosteroid preparations may retain efficacy with a significantly improved safety profile. The limited indications for antibiotics and probiotics have been further refined by recent data, although uncertainties remain. Advances in the understanding of thiopurine metabolism continue to improve dose optimization and the potential for deliberate therapeutic manipulation with adjunctive therapies. An improved knowledge of intracellular methotrexate metabolism may translate to similar opportunities in the future. This article discusses recent advances relevant to clinical practice today.
Similar content being viewed by others
References and Recommended Reading
Sandborn WJ: Current directions in IBD therapy: what goals are feasible with biological modifiers? Gastroenterology 2008, 135:1442–1447.
Vermeire S, van Assche G, Rutgeerts P: Review article: altering the natural history of Crohn’s disease-evidence for and against current therapies. Aliment Pharmacol Ther 2007, 25:3–12.
Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al.: The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology 2001, 121:255–260.
Kane SV, Cohen RD, Aikens JE, et al.: Prevalence of nonadherence with maintenance mesalamine in quiescent ulcerative colitis. Am J Gastroenterol 2001, 96:2929–2933.
Higgins PD, Rubin DT, Kaulback K, et al.: Systematic review: impact of non-adherence to 5-aminosalicylic acid products on the frequency and cost of ulcerative colitis flares. Aliment Pharmacol Ther 2009, 29:247–257.
Lichtenstein GR, Kamm MA, Boddu P, et al.: Effect of once- or twice-daily MMX mesalamine (SPD476) for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol 2007, 5:95–102.
Kamm MA, Sandborn WJ, Gassull M, et al.: Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology 2007, 132:66–75; quiz 432–433.
Kamm MA, Lichtenstein GR, Sandborn WJ, et al.: Randomised trial of once- or twice-daily MMX mesalazine for maintenance of remission in ulcerative colitis. Gut 2008, 57:893–902.
Kruis W, Kiudelis G, Racz I, et al.: Once daily versus three times daily mesalazine granules in active ulcerative colitis: a double-blind, double-dummy, randomised, non-inferiority trial. Gut 2009, 58:233–240.
Dignass AU, Bokemeyer B, Adamek H, et al.: Mesalamine once daily is more effective than twice daily in patients with quiescent ulcerative colitis. Clin Gastroenterol Hepatol 2009, 7:762–769.
Scherl EJ, Pruitt R, Gordon GL, et al.: Safety and efficacy of a new 3.3 g b.i.d. tablet formulation in patients with mild-to-moderately-active ulcerative colitis: a multicenter, randomized, double-blind, placebo-controlled study. Am J Gastroenterol 2009, 104:1452–1459.
Kane S, Lu F, Kornbluth A, et al.: Controversies in mucosal healing in ulcerative colitis. Inflamm Bowel Dis 2009, 15:796–800.
Rutgeerts P, Sandborn WJ, Feagan BG, et al.: Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005, 353:2462–2476.
Hanauer SB, Sandborn WJ, Kornbluth A, et al.: Delayedrelease oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: the ASCEND II trial. Am J Gastroenterol 2005, 100:2478–2485.
Hanauer SB, Sandborn WJ, Dallaire C, et al.: Delayedrelease oral mesalamine 4.8 g/day (800 mg tablets) compared to 2.4 g/day (400 mg tablets) for the treatment of mildly to moderately active ulcerative colitis: the ASCEND I trial. Can J Gastroenterol 2007, 21:827–834.
Sartor RB: Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 2004, 126:1620–1633.
Rubin DT, Kornblunth A: Role of antibiotics in the management of inflammatory bowel disease: a review. Rev Gastroenterol Disord 2005, 5 Suppl 3:S10–S15.
Isaacs K, Herfarth H: Role of probiotic therapy in IBD. Inflamm Bowel Dis 2008, 14:1597–1605.
Selby W, Pavli P, Crotty B, et al.: Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn’s disease. Gastroenterology 2007, 132:2313–2319.
Thia KT, Mahadevan U, Feagan BG, et al.: Ciprofloxacin or metronidazole for the treatment of perianal fistulas in patients with Crohn’s disease: a randomized, doubleblind, placebo-controlled pilot study. Inflamm Bowel Dis 2009, 15:17–24.
D’Haens GR, Vermeire S, Van Assche G, et al.: Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn’s disease: a controlled randomized trial. Gastroenterology 2008, 135:1123–1129.
Baker DE: Rifaximin: a nonabsorbed oral antibiotic. Rev Gastroenterol Disord 2005, 5:19–30.
Prantera C, Lochs H, Campieri M, et al.: Antibiotic treatment of Crohn’s disease: results of a multicentre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment Pharmacol Ther 2006, 23:1117–1125.
Isaacs KL, Sandler RS, Abreu M, et al.: Rifaximin for the treatment of active pouchitis: a randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis 2007, 13:1250–1255.
Shen B, Remzi FH, Lopez AR, et al.: Rifaximin for maintenance therapy in antibiotic-dependent pouchitis. BMC Gastroenterol 2008, 8:26.
Gionchetti P, Rizzello F, Venturi A, et al.: Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 2000, 119:305–309.
Kruis W, Fric P, Pokrotnieks J, et al.: Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53:1617–1623.
Rahimi R, Nikfar S, Rahimi F, et al.: A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn’s disease. Dig Dis Sci 2008, 53:2524–2531.
Sood A, Midha V, Makharia GK, et al.: The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol 2009.
Irving PM, Gearry RB, Sparrow MP, et al.: Review article: appropriate use of corticosteroids in Crohn’s disease. Aliment Pharmacol Ther 2007, 26:313–329.
Rhodes JM, Robinson R, Beales I, et al.: Clinical trial: oral prednisolone metasulfobenzoate (Predocol) vs. oral prednisolone for active ulcerative colitis. Aliment Pharmacol Ther 2008, 27:228–240.
Bossa F, Latiano A, Rossi L, et al.: Erythrocyte-mediated delivery of dexamethasone in patients with mild-to-moderate ulcerative colitis, refractory to mesalamine: a randomized, controlled study. Am J Gastroenterol 2008, 103:2509–2516.
Sandborn WJ: Azathioprine: state of the art in inflammatory bowel disease. Scand J Gastroenterol Suppl 1998, 225:92–99.
Gisbert JP, Nino P, Cara C, et al.: Comparative effectiveness of azathioprine in Crohn’s disease and ulcerative colitis: prospective, long-term, follow-up study of 394 patients. Aliment Pharmacol Ther 2008, 28:228–238.
Punati J, Markowitz J, Lerer T, et al.: Effect of early immunomodulator use in moderate to severe pediatric Crohn disease. Inflamm Bowel Dis 2008, 14:949–954.
Froslie KF, Jahnsen J, Moum BA, et al.: Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 2007, 133:412–422.
Mantzaris GJ, Christidou A, Sfakianakis M, et al.: Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohn’s disease. Inflamm Bowel Dis 2009, 15:375–382.
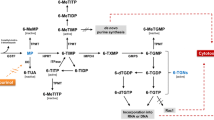
Dubinsky MC, Lamothe S, Yang HY, et al.: Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 2000, 118:705–713.
Osterman MT, Kundu R, Lichtenstein GR, et al.: Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology 2006, 130:1047–1053.
Weinshilboum RM, Sladek SL: Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet 1980, 32:651–662.
Gardiner SJ, Gearry RB, Begg EJ, et al.: Thiopurine dose in intermediate and normal metabolizers of thiopurine methyltransferase may differ three-fold. Clin Gastroenterol Hepatol 2008, 6:654–660; quiz 604.
Ansari A, Arenas M, Greenfield SM, et al.: Prospective evaluation of the pharmacogenetics of azathioprine in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther 2008, 28:973–983.
Marinaki AM, Ansari A, Duley JA, et al.: Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics 2004, 14:181–187.
Smith M, Marinaki A, Arenas M, et al.: Novel pharmacogenetic markers for treatment outcome in azathioprine-treated inflammatory bowel disease. Aliment Pharmacol Ther 2009, 30:375–384.
Gearry RB, Barclay ML, Roberts RL, et al.: Thiopurine methyltransferase and 6-thioguanine nucleotide measurement: early experience of use in clinical practice. Intern Med J 2005, 35:580–585.
Roblin X, Serre-Debeauvais F, Phelip JM, et al.: 6-tioguanine monitoring in steroid-dependent patients with inflammatory bowel diseases receiving azathioprine. Aliment Pharmacol Ther 2005, 21:829–839.
Roblin X, Peyrin-Biroulet L, Phelip JM, et al.: A 6-thioguanine nucleotide threshold level of 400 pmol/8 × 10 erythrocytes predicts azathioprine refractoriness in patients with inflammatory bowel disease and normal TPMT activity. Am J Gastroenterol 2008, 103:3115–3122.
Roberts RL, Gearry RB, Bland MV, et al.: Trinucleotide repeat variants in the promoter of the thiopurine S-methyltransferase gene of patients exhibiting ultrahigh enzyme activity. Pharmacogenet Genomics 2008, 18:434–438.
de Boer NK, Wong DR, Jharap B, et al.: Dose-dependent influence of 5-aminosalicylates on thiopurine metabolism. Am J Gastroenterol 2007, 102:2747–2753.
Sparrow MP, Hande SA, Friedman S, et al.: Effect of allopurinol on clinical outcomes in inflammatory bowel disease nonresponders to azathioprine or 6-mercaptopurine. Clin Gastroenterol Hepatol 2007, 5:209–214.
Leung Y, Sparrow MP, Schwartz M, Hanauer SB: Longterm efficacy and safety of allopurinol and azathioprine or 6-mercaptopurine in patients with inflammatory bowel disease. J Crohn’s Colitis 2009, 3:162–167.
Witte TN, Ginsberg AL: Use of allopurinol with low-dose 6-mercaptopurine in inflammatory bowel disease to achieve optimal active metabolite levels: a review of four cases and the literature. Can J Gastroenterol 2008, 22:181–185.
Rahhal RM, Bishop WP: Initial clinical experience with allopurinol-thiopurine combination therapy in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2008, 14:1678–1682.
Ansari A, Elliott T, Baburajan B, et al.: Long-term outcome of using allopurinol co-therapy as a strategy for overcoming thiopurine hepatotoxicity in treating inflammatory bowel disease. Aliment Pharmacol Ther 2008 28:734–741.
Wahed M, Louis-Auguste JR, Baxter LM, et al.: Efficacy of methotrexate in Crohn’s disease and ulcerative colitis patients unresponsive or intolerant to azathioprine/mercaptopurine. Aliment Pharmacol Ther 2009 30:614–620.
Nathan DM, Iser JH, Gibson PR: A single center experience of methotrexate in the treatment of Crohn’s disease and ulcerative colitis: a case for subcutaneous administration. J Gastroenterol Hepatol 2008, 23:954–958.
Stamp L, Roberts R, Kennedy M, et al.: The use of low dose methotrexate in rheumatoid arthritis-are we entering a new era of therapeutic drug monitoring and pharmacogenomics? Biomed Pharmacother 2006, 60:678–687.
Brooks AJ, Begg EJ, Zhang M, et al.: Red blood cell methotrexate polyglutamate concentrations in inflammatory bowel disease. Ther Drug Monit 2007, 29:619–625.
Cacheux W, Seksik P, Lemann M, et al.: Predictive factors of response to cyclosporine in steroid-refractory ulcerative colitis. Am J Gastroenterol 2008, 103:637–642.
Maser EA, Deconda D, Lichtiger S, et al.: Cyclosporine and infliximab as rescue therapy for each other in patients with steroid-refractory ulcerative colitis. Clin Gastroenterol Hepatol 2008, 6:1112–1116.
Branche J, Cortot A, Bourreille A, et al.: Cyclosporine treatment of steroid-refractory ulcerative colitis during pregnancy. Inflamm Bowel Dis 2009, 15:1044–1048.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sparrow, M.P., Irving, P.M. & Hanauer, S.B. Optimizing conventional therapies for inflammatory bowel disease. Curr Gastroenterol Rep 11, 496–503 (2009). https://doi.org/10.1007/s11894-009-0075-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11894-009-0075-6