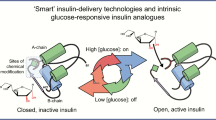
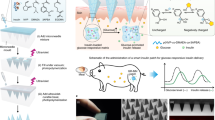
Abstract
Purpose of Review
The aim of this review is to summarize the development of the photoactivated depot (PAD) approach for the minimally invasive and continuously variable delivery of insulin.
Recent Findings
Using an insulin PAD, we have demonstrated that we can release native, bioactive insulin into diabetic animals in response to light signals from a small external LED light source. We have further shown that this released insulin retains bioactivity and reduces blood glucose. In addition, we have designed and constructed second generation materials that have high insulin densities, with the potential for multiple day delivery.
Summary
The PAD approach for insulin therapy holds promise for addressing the pressing need for continuously variable delivery methods that do not rely on pumps, and their myriad associated problems.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sonksen P, Sonksen J. Insulin: understanding its action in health and disease. Br J Anaesth. 2000;85(1):69–79.
Stephens E. Insulin therapy in type 1 diabetes. Med Clin N Am. 2015;99(1):145–56.
Switzer SM, Moser EG, Rockler BE, Garg SK. Intensive insulin therapy in patients with type 1 diabetes mellitus. Endocrinol Metab Clin N Am. 2012;41(1):89–104.
Kadish AH. Automation control of blood sugar. I. a servomechanism for glucose monitoring and control. Am J Med Electron. 1964;3:82–6.
Kadish AH. Automation control of blood sugar a servomechanism for glucose monitoring and control. Trans Am Soc Artif Intern Organs. 1963;9:363–7.
Battelino T, Omladiƒç JS, Phillip M. Closed loop insulin delivery in diabetes. Best Pract Res Clin Endocrinol Metab. 2015;29(3):315–25.
Davis T, Salahi A, Welsh JB, Bailey TS. Automated insulin pump suspension for hypoglycaemia mitigation: development, implementation and implications. Diabetes Obes Metab. 2015;17(12):1126–32.
Forlenza GP, Buckingham B, Maahs DM. Progress in diabetes technology: developments in insulin pumps, continuous glucose monitors, and Progress towards the artificial pancreas. J Pediatr. 2015.
Weinzimer SA. Closed-loop artificial pancreas: current studies and promise for the future. Current Opinion in Endocrinology, Diabetes and Obesity. 2012;19(2):88–92.
Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–20.
Kim J, Campbell AS, Wang J. Wearable non-invasive epidermal glucose sensors: a review. Talanta. 2018;177:163–70.
Siddiqui SA, Zhang Y, Lloret J, Song H, Obradovic Z. Pain-free blood glucose monitoring using wearable sensors: recent advancements and future prospects. IEEE Rev Biomed Eng [Article in Press]. 2018;11:21–35.
Boiroux D, Duun-Henriksen AK, Schmidt S, N√∏rgaard K, Poulsen NK, Madsen H, et al. Adaptive control in an artificial pancreas for people with type 1 diabetes. Control Engineering Practice. 2017;58:332–42.
Hajizadeh I, Rashid M, Samadi S, Feng J, Sevil M, Hobbs N, et al. Adaptive and personalized plasma insulin concentration estimation for artificial pancreas systems. J Diabetes Sci Technol. 2018;12:639–49.
Toffanin C, Visentin R, Messori M, Palma FD, Magni L, Cobelli C. Toward a run-to-run adaptive artificial pancreas: in silico results. IEEE Transactions on Biomedical Engineering. 2018;65(3):479–88.
Klonoff DC, Freckmann G, Heinemann L. Insulin pump occlusions: for patients who have been around the (infusion) block. J Diabetes Sci Technol. 2017;11(3):451–4.
Slover RH. Best ways and practices to avoid insulin pump catheter occlusions. Diabetes Technology and Therapeutics. 2016;18(3):118–9.
Heinemann L. Insulin infusion sets: a critical reappraisal. Diabetes Technology and Therapeutics. 2016;18(5):327–33.
Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting and research needs. A joint statement of the European Association for the Study of diabetes and the American Diabetes Association diabetes technology working group. Diabetologia. 2015;58(5):862–70.
Heinemann L, Krinelke L. Insulin infusion set: the Achilles heel of continuous subcutaneous insulin infusion. J Diabetes Sci Technol. 2012;6(4):954–64.
Heinemann L, Walsh J, Roberts R. We need more research and better designs for insulin infusion sets. J Diabetes Sci Technol. 2014;8(2):199–202.
Kuroda K, Takeshita Y, Kaneko S, Takamura T. Bending of a vertical cannula without alarm during insulin pump therapy as a cause of unexpected hyperglycemia: a Japanese issue? Journal of Diabetes Investigation. 2015;6(6):739–40.
Massa G, Gys I, Eyndt AO, Wauben K, Vanoppen A. Needle detachment from the sure-T¬Æ infusion set in two young children with diabetes mellitus (DM) treated with continuous subcutaneous insulin infusion (CSII) and unexplained hyperglycaemia. J Pediatr Endocrinol Metab. 2015;28(1–2):237–9.
Moser C, Maurer K, Binder E, Meraner D, Steichen E, Abt D, et al. Needle detachment in a slim and physically active child with insulin pump treatment. Pediatr Diabetes. 2015.
Plager P, Murati MA, Moran A, Sunni M. Two case reports of retained steel insulin pump infusion set needles. Pediatr Diabetes. 2015.
Jain PK, Karunakaran D, Friedman SH. Construction of a photoactivated insulin depot. Angew Chem Int Ed. 2013;52(5):1404–9. This was the first demonstration of the synthesis of insulin PAD materials, and showed that native insulin could be covalently linked to an insoluble resin, and then released in a controlled manner using light from an LED.
Sarode BR, Kover K, Tong PY, Zhang C, Friedman SH. Light control of insulin release and blood glucose using an injectable photoactivated depot. Mol Pharm. 2016;13(11):3835–41. This was the first in-vivo demonstration of the insulin PAD approach. It demonstrated in diabetic rats that sufficient light could cross the skin during transdermal irradiation to stimulate insulin release. It further showed that insulin was released into the blood rapidly (within five minutes) and maintained its bioactivity, effecting blood glucose reduction.
Nadendla K, Friedman SH. Light Control of Protein Solubility Through Isoelectric Point Modulation. J Am Chem Soc. 2017;139(49):17861–9.
Sarode BR, Jain PK, Friedman SH. Polymerizing Insulin with Photocleavable Linkers to Make Light-Sensitive Macropolymer Depot Materials. Macromol Biosci. 2016 Aug;16(9):1138–46.
Nadendla K, Sarode BR, Friedman SH. Hydrophobic Tags for Highly Efficient Light-Activated Protein Release. Mol Pharm. 2019.
Farhy LS, McCall AL. Glucagon -The new ‘insulin’ in the pathophysiology of diabetes. Current Opinion in Clinical Nutrition and Metabolic Care. 2015;18(5):407–14.
Haidar A, Legault L, Messier V, Miter TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: An open-label randomized controlled crossover trial. The Lancet Diabetes Endocrinol. 2015;3(2):17–26.
Gu Z, Aimetti AA, Wang Q, Dang TT, Zhang Y, Veiseh O, et al. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 2013 2013/05/28/;7 (6):4194–201.
Rege NK, Phillips NFB, Weiss MA. Development of glucose-responsive ‘smart’ insulin systems. Curr Opin Endocrinol Diabetes Obes. 2017;24(5):267–78.
Acknowledgments
The work reviewed in this article was largely executed by a group of talented and dedicated graduate students. They are: Piyush K. Jain, Dipu Karunakaran, Bhagyesh R. Sarode, Karthik Nadendla, Swetha Chintala, Parth Shah and Mayank Sharma.
In addition all animal studies were performed in collaboration with Professor Karen Kover (Childrens’ Mercy Hospital, Kansas City).
The work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number DP3DK106921 as well as the support of a University of Missouri Fast Track Award and the UMKC School of Pharmacy Dean’s Bridge Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Simon H. Friedman has a patent issued (US 10159735), and a patent pending (on Drug Conjugates with Photocleavable Solubility Modulators).
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Therapies and New Technologies in the Treatment of Diabetes
Rights and permissions
About this article
Cite this article
Friedman, S.H. Replacing Pumps with Light Controlled Insulin Delivery. Curr Diab Rep 19, 122 (2019). https://doi.org/10.1007/s11892-019-1233-3
Published:
DOI: https://doi.org/10.1007/s11892-019-1233-3