Abstract
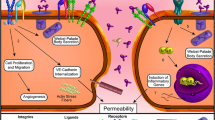
Tie2 is a tyrosine kinase receptor located predominantly on vascular endothelial cells that plays a central role in vascular stability. Angiopoietin-1 (Angpt1), produced by perivascular cells, binds, clusters, and activates Tie2, leading to Tie2 autophosphorylation and downstream signaling. Activated Tie2 increases endothelial cell survival, adhesion, and cell junction integrity, thereby stabilizing the vasculature. Angiopoietin-2 (Angpt2) and vascular endothelial-protein tyrosine phosphatase (VE-PTP) are negative regulators increased by hypoxia; they inactivate Tie2, destabilizing the vasculature and increasing responsiveness to vascular endothelial growth factor (VEGF) and other inflammatory cytokines that stimulate vascular leakage and neovascularization. AKB-9778 is a small-molecule antagonist of VE-PTP which increases phosphorylation of Tie2 even in the presence of high Angpt2 levels. In preclinical studies, AKB-9778 reduced VEGF-induced leakage and ocular neovascularization (NV) and showed additive benefit when combined with VEGF suppression. In two clinical trials in diabetic macular edema (DME) patients, subcutaneous injections of AKB-9778 were safe and provided added benefit to VEGF suppression. Preliminary data suggest that AKB-9778 monotherapy improves diabetic retinopathy. These data suggest that Tie2 activation may be a valuable strategy to treat or prevent diabetic retinopathy.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of Importance •• Of major importance
Klein R, Klein B. Vision disorders in diabetes. In: Group NDD, editor. Diabetes in America. 2nd ed. Washington: National Institutes of Health; 1995.
Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67–81.
Campochiaro PA, Aiello LP, Rosenfeld PJ. Anti-vascular endothelial growth factor agents in the treatment of retinal disease. From bench to bedside. Ophthalmology 2016; In press.
Dumont DJ, Yamaguchi TP, Conlon RA, et al. tek, a novel tyrosine kinase gen located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene. 1992;7:1471–80.
Partanen J, Armstrong E, Makela TP, et al. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol. 1992;12:1698–707.
Dumont DJ, Gradwohl G, Fong G-H, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–909.
Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–4.
Davis S, Aldrich TH, Jones P, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–9.
Procopio WN, Pelavin PI, Lee WMF, et al. Angiopoeitin-1 and -2 coiled coil domains mediate distinct homo-oliomerization patterns, but fibrinogen-like domains mediate ligand activity. J Biol Chem. 1999;274:30196–201.
Suri C, Jones PF, Patan S, et al. Requisite role of Angiopoietin-1, a ligand for the Tie2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80.
Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60.
Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Devel Cell. 2002;3:411–23.
Valenzuela DM, Griffiths JA, Rojas J, et al. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci U S A. 1999;96:1904–9.
Fachinger G, Deutsch U, Risau W. Functional interaction of vascular endothelial-protein-tyrosine phosphatase with the angiopoietin receptor Tie-2. Oncogene. 1999;18:5948–3.
Krueger NX, Streuli M, Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990;9:3241–52.
Wong AL, Haroon ZA, Werner S, et al. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res. 1997;81:567–74.
Mandriota SJ, Pepper MS. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res. 1998;83:852–9.
Oh H, Takagi H, Suzuma K, et al. Hypoxia and vascular endothelial growth factor selectively upregulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem. 1999;274:15732–9.
Yacyshyn OK, Lai PFH, Forse K, et al. Tyrosine phosphatase beta regulates angiopoietin-Tie2 signaling in human endothelial cells. Angiogenesis. 2009;12:25–33.
•• Shen J, Frye M, Lee BL, et al. Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J Clin Invest. 2014;124:4564–76. This study demonstrates the critical role of VE-PTP in regulation of Tie2 and the potential of using VE-PTP inhibitors to activate Tie2 and reduce vascular responsiveness to VEGF.
Kontos CD, Stauffer TP, Yang W-P, et al. Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol Cell Biol. 1998;11:4131–40.
Papapetropoulos A, Garcia-Cardena G, Dengler TJ, et al. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999;79:213–23.
Kim I, Kim HG, So J-N, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–9.
Kim I, Kim JH, Moon SO, et al. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene. 2000;19:4549–52.
Yuan HT, Khankin EV, Karumanchi SA, et al. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Molec Cell Biol. 2009;29:2011–22.
Tai L-K, Zheng Q, Pan S, et al. Flow activates ERK1/2 and endothelial nitric oxide synthase via a pathway involving PECAM1, SHP2, and Tie2. J Biol Chem. 2005;280:29620–4.
Szmitko PE, Wang CH, Weisel RD, et al. New markers of inflammation and endothelial cell activation: part I. Circulation. 2003;108:1917–23.
Fukuhara S, Sako K, Minami T, et al. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–26.
Saharinen P, Eklund L, Miettinen J, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–37.
Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Devel Cell. 2008;14:25–36.
Wittchen ES, Worthylake RA, Kelly P, et al. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 2005;280:11675–82.
Mammoto T, Parikh SM, Mammoto A, et al. Angiopoietin-1 requires p190 rhoGAP to protect against vascular leakage in vivo. J Biol Chem. 2007;282:23910–8.
David S, Ghosh CC, Mukherjee A, et al. Angiopoietin-1 requires IQ domain GTPase-activating protein 1 to activate Rac1 and promote endothelial barrier defense. Arterioscler Thromb Vasc Biol. 2011;31:2643–52.
•• Frye M, Dierkes M, Kuppers V, et al. Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J Exp Med. 2015;212:2267–87. This study demonstrates that suppression of VE-PTP stabilizes endothelial cell junctions independent of any effects on VE-cadherin.
Suri C, McClain J, Thurston G, et al. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–71.
Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–5.
Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–3.
Hackett SF, Ozaki H, Strauss RW, et al. Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol. 2000;184:275–84.
Hackett SF, Wiegand SJ, Yancopoulos G, et al. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192:182–7.
Okamoto N, Tobe T, Hackett SF, et al. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol. 1997;151:281–91.
Ohno-Matsui K, Hirose A, Yamamoto S, et al. Inducible expression of vascular endothelial growth factor in photoreceptors of adult mice causes severe proliferative retinopathy and retinal detachment. Am J Pathol. 2002;160:711–9.
Oshima Y, Deering T, Oshima S, et al. Angiopoietin-2 enhances retinal vessel sensitivity to vascular endothelial growth factor. J Cell Physiol. 2004;199:412–7.
Oshima Y, Oshima S, Nambu H, et al. Different effects of angiopoietin 2 in different vascular beds in the eye; new vessels are most sensitive. FASEB J. 2005;19:963–5.
Hammes HP, Wagner LJ, Feng Y, et al. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes. 2004;53:1104–10.
Uemura A, Ogawa M, Hirashima M, et al. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest. 2002;110:1619–28.
Enge M, Bjarnegard M, Gerhardt H, et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–16.
Nambu H, Nambu R, Oshima Y, et al. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther. 2004;11:865–73.
Nambu H, Umeda N, Kachi S, et al. Angiopoietin 1 prevents retinal detachment in an aggressive model of proliferative retinopathy, but has no effect on established neovascularization. J Cell Physiol. 2005;204:227–35.
Joussen AM, Poulaki V, Tsujikawa A, et al. Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol. 2002;160:1683–93.
Campochiaro PA, Hafiz G, Mir TA, et al. Pro-permeability factors in diabetic macular edema; the diabetic macular edema treated with Ozurdex study. Am J Ophthalmol. 2016. doi:10.1016/j.ajo.2016.04.017.
Foroogbian F, Kertes PJ, Eng KT, et al. Alterations in the intraocular cytokine milieu after intravitreal bevacizumab. Invest Ophthalmol Vis Sci. 2010;51:2388–92.
Loukovaara S, Robciuc A, Holopainen JM, et al. Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFbeta1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol. 2013;91:531–9.
Li JK, Wei F, Jin XH, et al. Changes in vitreous VEGF, bFGF and fibrosis in proliferative diabetic retinopathy after intravitreal bevacizumab. Int J Ophthalmol. 2015;8:1202–6.
Yoshimura T, Sonoda KH, Sugahara M, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4:e8158.
Kinnnen K, Puustjarvi T, Terasvirta M, et al. Differences in retinal neovascular tissue and vitreous humour in patients with type 1 and type 2 diabetes. Br J Ophthalmol. 2009;93:1109–15.
Watanabe D, Suzuma K, Suzuma I, et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2005;139:476–81.
• Campochiaro PA, Sophie R, Tolentino M, et al. Treatment of diabetic macular edema with an inhibitor of vascular endothelial-protein tyrosine phosphatase that activates Tie2. Ophthalmology. 2015;122:545–54. This study demonstrated that subcutaneous injections of AKB-9778, a small molecule competitive inhibitor of VE-PTP that activates Tie2, is safe and well tolerated in patients with diabetic macular edema (DME) and reduces edema in some, but not all patients with DME.
•• Campochiaro PA, Khanani A, Singer M, et al. Enhanced benefit in diabetic macular edema from AKB-9778 Tie2 activation combined with vascular endothelial growth factor suppression. Ophthalmology. 2016. doi:10.1016/j.ophtha.2016.04.025. This study demonstrated that the combination of subcutaneous injections of AKB-9778 and intravitreous injections of ranibizumab cause significantly greater reduction of DME than either alone. Preliminary data suggested that AKB-9778 monotherapy reduces severity of background diabetic retinopathy.
Ip MS, Domalpally A, Hopkins JJ, et al. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130:1145–52.
Lee JS, Song SH, Kim JM, et al. Angiopoietin-1 prevents hypertension and target organ damage through its interaction with endothelial Tie2 receptor. Cardiovasc Res. 2008;78:572–80.
Jeansson M, Gawlik A, Anderson G, et al. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest. 2011;121:2278–89.
Lee S, Kim W, Moon SO, et al. Renoprotective effect of COMP-angiopoietin-1 in db/db mice with type 2 diabetes. Nephrol Dial Transplant. 2007;22:396–408.
Shyu KG, Manor O, Magner M, et al. Direct intramuscular injection of plasmid DNA encoding angiopoietin-1 but not angiopoietin-2 augments revascularization in the rabbit hindlimb. Circulation. 1998;98:2081–1087.
Ye L, Haider HK, Jinag S, et al. Improved angiogenic response in pig heart following ischaemic injury using human skeletal myoblast simultaneously expressing VEGF-165 and angiopoietin-1. Eur J Heart Fail. 2007;9:15–22.
Chen JX, Stinnett A. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28:1606–13.
Chen JX, Stinnett A. Ang-1 gene therapy inhibits hypoxia-inducible factor-1alpha (HIF-1alpha)-prolyl-4-hydroxylase-2, stabilizes HIF-1alpha expression, and normalizes immature vasculature in db/db mice. Diabetes. 2008;57:3335–43.
Chae JK, Kim I, Lim ST, et al. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol. 2000;20:2573–8.
Tuo QH, Zeng H, Stinnett A, et al. Critical role of angiopoietins/Tie-2 in hyperglycemic exacerbation of myocardial infarction and impaired angiogenesis. Am J Physiol Heart Circ Physiol. 2008;294:H2547–57.
Kampfer H, Pfeilschifter J, Frank S. Expressional regulation of angiopoietin-1 and -2 and the tie-1 and -2 receptor tyrosine kinases during cutaneous wound healing: a comparative study of normal and impaired repair. Lab Invest. 2001;81:361–73.
Cho CH, Sung HK, Kim KT, et al. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci U S A. 2006;103:4946–51.
Bitto A, Minutoli L, Galeano MR, et al. Angiopoietin-1 gene transfer improves impaired wound healing in genetically diabetic mice without increasing VEGF expression. Clin Sci (Lond). 2008;114:707–18.
Kosacka J, Nowicki M, Kloting N, et al. COMP-angiopoietin-1 recovers molecular biomarkers of neuropathy and improves vascularisation in sciatic nerve of ob/ob mice. PLoS One. 2012;7:e32881.
Jin HR, Kim WJ, Song JS, et al. Intracavernous delivery of a designed angiopoietin-1 variant rescues erectile function by enhancing endothelial regeneration in the streptozotocin-induced diabetic mouse. Diabetes. 2011;60:969–80.
Anuradha S, Mohan V, Gokulakrishnan K, et al. Angiopoietin-2 levels in glucose intolerance, hypertension, and metabolic syndrome in Asian Indians (Chennai Urban Rural Epidemiology Study-74). Metabolism. 2010;59:774–9.
Lieb W, Zachariah JP, Xanthakis V, et al. Clinical and genetic correlates of circulating angiopoietin-2 and soluble Tie-2 in the community. Circ Cardiovasc Genet. 2010;3:300–6.
Lim HS, Blann AD, Chong AY, et al. Plasma vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in diabetes: implications for cardiovascular risk and effects of multifactorial intervention. Diabetes Care. 2004;27:2918–24.
Lip PL, Chatterjee S, Caine GJ, et al. Plasma vascular endothelial growth factor, angiopoietin-2, and soluble angiopoietin receptor tie-2 in diabetic retinopathy: effects of laser photocoagulation and angiotensin receptor blockade. Br J Ophthalmol. 2004;88:1543–6.
Rasul S, Reiter MH, Ilhan A, et al. Circulating angiopoietin-2 and soluble Tie-2 in type 2 diabetes mellitus: a cross-sectional study. Cardiovasc Diabetol. 2011;10:55.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Peter A. Campochiaro reports grants and other from Aerpio Therapeutics, grants and other from Regeneron Therapeutics, grants and other from Genentech/Roche, grants and personal fees from Alimera, grants and personal fees from Allergan, personal fees from Applied Genetic Technologies, grants and personal fees from AsclepiX, grants and personal fees from Rxi, personal fees from Allegro, personal fees from Intrexon, grants and personal fees from Regenxbio, grants from AbbVie, grants from Genzyme, grants from GlaxoSmithKline, grants from Oxford Biomedica, personal fees and other from Graybug, and personal fees from Merck.
Kevin G. Peters is an employee of Aerpio Therapeutics. In addition, Dr. Peters is an inventor on issued and pending composition of matter and methods of use patents for AKB-9778.
Human and Animal Rights and Informed Consent
The clinical trials discussed in this manuscript were approved by the appropriate institutional and/or national research ethics committee and were performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
All procedures performed in studies involving animals were in accordance with the ethical standards of the Johns Hopkins University School of Medicine where the studies were conducted.
Additional information
This article is part of the Topical Collection on Microvascular Complications—Retinopathy
Rights and permissions
About this article
Cite this article
Campochiaro, P.A., Peters, K.G. Targeting Tie2 for Treatment of Diabetic Retinopathy and Diabetic Macular Edema. Curr Diab Rep 16, 126 (2016). https://doi.org/10.1007/s11892-016-0816-5
Published:
DOI: https://doi.org/10.1007/s11892-016-0816-5