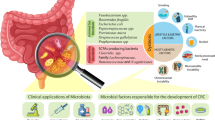
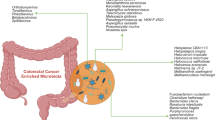
Abstract
Purpose of review
There is growing evidence to suggest that gut microbiota plays an important role in colorectal carcinogenesis. Western diet is associated with gut microbial dysbiosis, which leads to inflammation, oxidative stress, and genotoxic effects, all common risk factors for colorectal cancer.
Recent findings
Fusobacterium nucleatum, Helicobacter pylori, Bacteroides fragilis, Escherichia coli, and Streptococcus bovis are the main bacterial species associated with colorectal carcinogenesis. Gut microbiota transforms both diet- (meat, processed meat products, fat) and host (bile acids)-derived precursors into carcinogens and further interferes with anti-cancer drug metabolism, chemotherapy efficacy, and drug-induced toxicity. Nutritional interventions, as well as the administration of beneficial bacteria (probiotics), dietary fiber (including prebiotics) supplements, and synbiotics (probiotic + prebiotic), may reduce the risk of colorectal cancer and side effects of anti-cancer therapy.
Summary
Current evidence suggests gut microbiota may predispose or protect against colorectal cancer. Restoring gut microbial dysbiosis is an emerging nutritional and clinical target in oncology.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. https://doi.org/10.1002/ijc.29210.
R John HR. The global economic cost of cancer: a report summary. Atlanta American Cancer Society 2014.
Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. https://doi.org/10.1146/annurev-pathol-011110-130235.
Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–502. https://doi.org/10.1016/S0140-6736(13)61649-9.
Karunanithi S, Levi L, DeVecchio J, Karagkounis G, Reizes O, Lathia JD, et al. RBP4-STRA6 pathway drives cancer stem cell maintenance and mediates high-fat diet-induced colon carcinogenesis. Stem Cell Reports. 2017;9(2):438–50. https://doi.org/10.1016/j.stemcr.2017.06.002.
Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800–12. https://doi.org/10.1038/nrc3610.
Gagniere J, Raisch J, Veziant J, Barnich N, Bonnet R, Buc E, et al. Gut microbiota imbalance and colorectal cancer. World J Gastroenterol. 2016;22(2):501–18. https://doi.org/10.3748/wjg.v22.i2.501.
Savin Z, Kivity S, Yonath H, Yehuda S. Smoking and the intestinal microbiome. Arch Microbiol. 2018;200(5):677–84. https://doi.org/10.1007/s00203-018-1506-2.
Walker AW, Lawley TD. Therapeutic modulation of intestinal dysbiosis. Pharmacol Res. 2013;69(1):75–86. https://doi.org/10.1016/j.phrs.2012.09.008.
Sato J, Kanazawa A, Ikeda F, Yoshihara T, Goto H, Abe H, et al. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care. 2014;37(8):2343–50. https://doi.org/10.2337/dc13-2817.
Dulal S, Keku TO. Gut microbiome and colorectal adenomas. Cancer Journal (Sudbury, Mass). 2014;20(3):225–31. https://doi.org/10.1097/ppo.0000000000000050.
Hawrelak JA, Myers SP. The causes of intestinal dysbiosis: a review. Alternat Med Rev. 2004;9(2):180–97.
Tilg H, Adolph TE, Gerner RR, Moschen AR. The intestinal microbiota in colorectal cancer. Cancer Cell. 2018;33(6):954–64. https://doi.org/10.1016/j.ccell.2018.03.004.
Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533. https://doi.org/10.1371/journal.pbio.1002533.
Mc CW, Mason JM 3rd. Enterococcal endocarditis associated with carcinoma of the sigmoid: report of a case. J Med Assoc State Ala. 1951;21(6):162–6.
Moore WE, Moore LH. Intestinal floras of populations that have a high risk of colon cancer. Appl Environ Microbiol. 1995;61(9):3202–7.
Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260–70. https://doi.org/10.1038/nrg3182.
• Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ (Clinical research ed). 2018;361:k2179. https://doi.org/10.1136/bmj.k2179 This review provides a background on gut microbiota and health.
Sheflin AM, Whitney AK, Weir TL. Cancer-promoting effects of microbial dysbiosis. Curr Oncol Rep. 2014;16(10):406. https://doi.org/10.1007/s11912-014-0406-0.
Zitvogel L, Galluzzi L, Viaud S, Vetizou M, Daillere R, Merad M et al. Cancer and the gut microbiota: an unexpected link. Science translational medicine. 2015;7(271):271ps1. doi:https://doi.org/10.1126/scitranslmed.3010473.
• Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology. 2017;153(6):1621–33.e6. https://doi.org/10.1053/j.gastro.2017.08.022 First study providing evidence for the direct pro-tumorigenic effect of the CRC microbiota in mouse models.
Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206. https://doi.org/10.1016/j.chom.2013.07.012.
Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, et al. Binding of the Fap2 protein of fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42(2):344–55. https://doi.org/10.1016/j.immuni.2015.01.010.
Quah SY, Bergenholtz G, Tan KS. Fusobacterium nucleatum induces cytokine production through toll-like-receptor-independent mechanism. Int Endod J. 2014;47(6):550–9. https://doi.org/10.1111/iej.12185.
Park SR, Kim DJ, Han SH, Kang MJ, Lee JY, Jeong YJ, et al. Diverse toll-like receptors mediate cytokine production by fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans in macrophages. Infect Immun. 2014;82(5):1914–20. https://doi.org/10.1128/iai.01226-13.
Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105(24):1907–11. https://doi.org/10.1093/jnci/djt300.
Ito M, Kanno S, Nosho K, Sukawa Y, Mitsuhashi K, Kurihara H, et al. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int J Cancer. 2015;137(6):1258–68. https://doi.org/10.1002/ijc.29488.
Li YY, Ge QX, Cao J, Zhou YJ, Du YL, Shen B, et al. Association of Fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World J Gastroenterol. 2016;22(11):3227–33. https://doi.org/10.3748/wjg.v22.i11.3227.
Guo S, Li L, Xu B, Li M, Zeng Q, Xiao H, et al. A simple and novel fecal biomarker for colorectal cancer: ratio of fusobacterium nucleatum to probiotics populations, based on their antagonistic effect. Clin Chem. 2018;64:1327–37. https://doi.org/10.1373/clinchem.2018.289728.
Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345(2):196–202. https://doi.org/10.1016/j.canlet.2013.08.016.
Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans. 2012;100(Pt E):1–538.
Tatishchev SF, Vanbeek C, Wang HL. Helicobacter pylori infection and colorectal carcinoma: is there a causal association? J Gastrointest Oncol. 2012;3(4):380–5. https://doi.org/10.3978/j.issn.2078-6891.2012.058.
Kapetanakis N, Kountouras J, Zavos C, Anastasiadou K, Tsarouchas G, Michael S, et al. Potential oncogenic properties of mobilized stem cells in a subpopulation of inflammatory bowel disease patients infected with Helicobacter pylori. Inflamm Bowel Dis. 2013;19(2):E27–9. https://doi.org/10.1002/ibd.22911.
Zhao Y, Wang X, Wang Y. Helicobacter pylori infection and colorectal carcinoma risk: a meta-analysis. J Cancer Res Ther. 2016;12(Supplement):15–8. https://doi.org/10.4103/0973-1482.191621.
Epplein M, Pawlita M, Michel A, Peek RM Jr, Cai Q, Blot WJ. Helicobacter pylori protein-specific antibodies and risk of colorectal cancer. Cancer Epidemiol Biomark Prev. 2013;22(11):1964–74. https://doi.org/10.1158/1055-9965.epi-13-0702.
Jones KR, Whitmire JM, Merrell DS. A tale of two toxins: helicobacter pylori CagA and VacA modulate host pathways that impact disease. Front Microbiol. 2010;1:115. https://doi.org/10.3389/fmicb.2010.00115.
Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin Infect Dis. 2015;60(2):208–15. https://doi.org/10.1093/cid/ciu787.
Keenan JI, Aitchison A, Purcell RV, Greenlees R, Pearson JF, Frizelle FA. Screening for enterotoxigenic Bacteroides fragilis in stool samples. Anaerobe. 2016;40:50–3. https://doi.org/10.1016/j.anaerobe.2016.05.004.
Sears CL, Geis AL, Housseau F. Bacteroides fragilis subverts mucosal biology: from symbiont to colon carcinogenesis. J Clin Invest. 2014;124(10):4166–72. https://doi.org/10.1172/jci72334.
Chung L, Thiele Orberg E, Geis AL, Chan JL, Fu K, DeStefano Shields CE, et al. Bacteroides fragilis toxin coordinates a pro-carcinogenic inflammatory cascade via targeting of colonic epithelial cells. Cell Host Microbe. 2018;23(2):203–14 e5. https://doi.org/10.1016/j.chom.2018.01.007.
Wassenaar TM. E. coli and colorectal cancer: a complex relationship that deserves a critical mindset. Crit Rev Microbiol. 2018;44:1–14. https://doi.org/10.1080/1040841x.2018.1481013.
Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, et al. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PloS One. 2013;8(2):e56964. https://doi.org/10.1371/journal.pone.0056964.
Yang Y, Jobin C. Microbial imbalance and intestinal pathologies: connections and contributions. Dis Model Mech. 2014;7(10):1131–42. https://doi.org/10.1242/dmm.016428.
Feng Q, Liang S, Jia H, Stadlmayr A, Tang L, Lan Z, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6:6528. https://doi.org/10.1038/ncomms7528.
Schlegel L, Grimont F, Ageron E, Grimont PA, Bouvet A. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int J Syst Evol Microbiol. 2003;53(Pt 3):631–45. https://doi.org/10.1099/ijs.0.02361-0.
Abdulamir AS, Hafidh RR, Abu BF. The association of Streptococcus bovis/gallolyticus with colorectal tumors: the nature and the underlying mechanisms of its etiological role. J Exp Clin Cancer Res: CR. 2011;30:11. https://doi.org/10.1186/1756-9966-30-11.
Krishnan S, Eslick GD. Streptococcus bovis infection and colorectal neoplasia: a meta-analysis. Color Dis. 2014;16(9):672–80. https://doi.org/10.1111/codi.12662.
Pasquereau-Kotula E, Martins M, Aymeric L, Dramsi S. Significance of Streptococcus gallolyticus subsp. gallolyticus association with colorectal cancer. Front Microbiol. 2018;9:614. https://doi.org/10.3389/fmicb.2018.00614.
Aymeric L, Donnadieu F, Mulet C, du Merle L, Nigro G, Saffarian A, et al. Colorectal cancer specific conditions promote Streptococcus gallolyticus gut colonization. Proc Natl Acad Sci U S A. 2018;115(2):E283–E91. https://doi.org/10.1073/pnas.1715112115.
de Almeida CV, Taddei A, Amedei A. The controversial role of Enterococcus faecalis in colorectal cancer. Ther Adv Gastroenterol. 2018;11:1756284818783606. https://doi.org/10.1177/1756284818783606.
Clemente JC, Manasson J, Scher JU. The role of the gut microbiome in systemic inflammatory disease. BMJ (Clinical research ed). 2018;360:j5145. https://doi.org/10.1136/bmj.j5145.
Forbes JD, Van Domselaar G, Bernstein CN. The gut microbiota in immune-mediated inflammatory diseases. Front Microbiol. 2016;7:1081. https://doi.org/10.3389/fmicb.2016.01081.
Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol. 2016;22(20):4794–801. https://doi.org/10.3748/wjg.v22.i20.4794.
• Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–72. https://doi.org/10.1038/nrmicro3344 This article details the role of bacterial metabolites on CRC.
•• Nistal E, Fernandez-Fernandez N, Vivas S, Olcoz JL. Factors determining colorectal cancer: the role of the intestinal microbiota. Front Oncol. 2015;5:220. https://doi.org/10.3389/fonc.2015.00220 This article highlights how pro-carcinogenic compounds are related with CRC.
Aune D, Lau R, Chan DS, Vieira R, Greenwood DC, Kampman E, et al. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology. 2011;141(1):106–18. https://doi.org/10.1053/j.gastro.2011.04.013.
Schwingshackl L, Schwedhelm C, Hoffmann G, Knuppel S, Laure Preterre A, Iqbal K, et al. Food groups and risk of colorectal cancer. Int J Cancer. 2018;142(9):1748–58. https://doi.org/10.1002/ijc.31198.
Wu QJ, Yang Y, Vogtmann E, Wang J, Han LH, Li HL, et al. Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann Oncol. 2013;24(4):1079–87. https://doi.org/10.1093/annonc/mds601.
Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011;6(6):e20456. https://doi.org/10.1371/journal.pone.0020456.
Cai S, Li Y, Ding Y, Chen K, Jin M. Alcohol drinking and the risk of colorectal cancer death: a meta-analysis. Eur J Cancer Prev. 2014;23(6):532–9. https://doi.org/10.1097/cej.0000000000000076.
Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, et al. Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive E coli infection and intestinal inflammation. Sci Rep. 2016;6:19032. https://doi.org/10.1038/srep19032 https://www.nature.com/articles/srep19032#supplementary-information.
Mehta RS, Nishihara R, Cao Y, Song M, Mima K, Qian ZR, et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by Fusobacterium nucleatum in tumor tissue. JAMA oncology. 2017;3(7):921–7. https://doi.org/10.1001/jamaoncol.2016.6374.
Casterline BW, Hecht AL, Choi VM, Bubeck WJ. The Bacteroides fragilis pathogenicity island links virulence and strain competition. Gut Microbes. 2017;8(4):374–83. https://doi.org/10.1080/19490976.2017.1290758.
Christ A, Gunther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 2018;172(1–2):162–175 e14. doi:https://doi.org/10.1016/j.cell.2017.12.013.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. https://doi.org/10.1038/nature12820.
Red meat and processed meat Lyon FR: International Agency for Research on Cancer 2018. For more information contact publications@iarc.fr.; 2018.
Turner ND, Lloyd SK. Association between red meat consumption and colon cancer: a systematic review of experimental results. Experimental biology and medicine (Maywood, NJ). 2017;242(8):813–39. doi:https://doi.org/10.1177/1535370217693117.
• Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17(5):271–85. https://doi.org/10.1038/nrc.2017.13 Overview of the microbiota ability to modulate anti-cancer treatment.
• Jobin C. Precision medicine using microbiota. Science. 2018;359(6371):32 New considerations about microbiota and immunotherapy.
Westman EL, Canova MJ, Radhi IJ, Koteva K, Kireeva I, Waglechner N, et al. Bacterial inactivation of the anticancer drug doxorubicin. Chem Biol. 2012;19(10):1255–64. https://doi.org/10.1016/j.chembiol.2012.08.011.
Okuda H, Ogura K, Kato A, Takubo H, Watabe T. A possible mechanism of eighteen patient deaths caused by interactions of sorivudine, a new antiviral drug, with oral 5-fluorouracil prodrugs. J Pharmacol Exp Ther. 1998;287(2):791–9.
Kim DH, Jin YH. Intestinal bacterial beta-glucuronidase activity of patients with colon cancer. Arch Pharm Res. 2001;24(6):564–7.
Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Keefe DM. Faecal microflora and beta-glucuronidase expression are altered in an irinotecan-induced diarrhea model in rats. Cancer Biol Ther. 2008;7(12):1919–25.
Lin XB, Dieleman LA, Ketabi A, Bibova I, Sawyer MB, Xue H, et al. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS One. 2012;7(7):e39764. https://doi.org/10.1371/journal.pone.0039764.
Dabek M, McCrae SI, Stevens VJ, Duncan SH, Louis P. Distribution of beta-glucosidase and beta-glucuronidase activity and of beta-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol Ecol. 2008;66(3):487–95. https://doi.org/10.1111/j.1574-6941.2008.00520.x.
McIntosh FM, Maison N, Holtrop G, Young P, Stevens VJ, Ince J, et al. Phylogenetic distribution of genes encoding beta-glucuronidase activity in human colonic bacteria and the impact of diet on faecal glycosidase activities. Environ Microbiol. 2012;14(8):1876–87. https://doi.org/10.1111/j.1462-2920.2012.02711.x.
Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330(6005):831–5. https://doi.org/10.1126/science.1191175.
Lehouritis P, Cummins J, Stanton M, Murphy CT, McCarthy FO, Reid G, et al. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep. 2015;5:14554. https://doi.org/10.1038/srep14554.
Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–6. https://doi.org/10.1126/science.1240537.
Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–70. https://doi.org/10.1126/science.1240527.
Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity. 2016;45(4):931–43. https://doi.org/10.1016/j.immuni.2016.09.009.
Forsgård RA, Korpela R, Holma R, Lindén J, Frias R, Spillmann T, et al. Intestinal permeability to iohexol as an in vivo marker of chemotherapy-induced gastrointestinal toxicity in Sprague-Dawley rats. Cancer Chemother Pharmacol. 2016;78(4):863–74. https://doi.org/10.1007/s00280-016-3150-3.
Forsgård RA, Marrachelli VG, Korpela K, Frias R, Collado MC, Korpela R, et al. Chemotherapy-induced gastrointestinal toxicity is associated with changes in serum and urine metabolome and fecal microbiota in male Sprague-Dawley rats. Cancer Chemother Pharmacol. 2017;80(2):317–32. https://doi.org/10.1007/s00280-017-3364-z.
Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther 2014;40(5):409–421. doi:https://doi.org/10.1111/apt.12878.
Ó’Broin P, Vaitheesvaran B, Saha S, Hartil K, Chen EI, Goldman D, et al. Intestinal microbiota-derived metabolomic blood plasma markers for prior radiation injury. Int J Radiat Oncol*Biol*Phys. 2015;91(2):360–7. https://doi.org/10.1016/j.ijrobp.2014.10.023.
Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy--pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol. 2014;11(8):470–9. https://doi.org/10.1038/nrgastro.2014.46.
Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci U S A. 2005;102(37):13254–9. https://doi.org/10.1073/pnas.0504830102.
dos Reis SA, da Conceição LL, Siqueira NP, Rosa DD, da Silva LL, Peluzio MdCG. Review of the mechanisms of probiotic actions in the prevention of colorectal cancer. Nutr Res 2017;37:1–19. doi:https://doi.org/10.1016/j.nutres.2016.11.009.
Ambalam P, Raman M, Purama RK, Doble M. Probiotics, prebiotics and colorectal cancer prevention. Best Pract Res Clin Gastroenterol. 2016;30(1):119–31. https://doi.org/10.1016/j.bpg.2016.02.009.
Raman M, Ambalam P, Kondepudi KK, Pithva S, Kothari C, Patel AT, et al. Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut Microbes. 2013;4(3):181–92. https://doi.org/10.4161/gmic.23919.
• Garrett WS. Cancer and the microbiota. Science. 2015;348(6230):80 An important review of the relationship between microbiota and carcinogenesis.
Gao Z, Guo B, Gao R, Zhu Q, Wu W, Qin H. Probiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancer. Mol Med Rep. 2015;12(4):6119–27. https://doi.org/10.3892/mmr.2015.4124.
Odamaki T, Sugahara H, Yonezawa S, Yaeshima T, Iwatsuki K, Tanabe S, et al. Effect of the oral intake of yogurt containing Bifidobacterium longum BB536 on the cell numbers of enterotoxigenic Bacteroides fragilis in microbiota. Anaerobe. 2012;18(1):14–8. https://doi.org/10.1016/j.anaerobe.2011.11.004.
Golkhalkhali B, Rajandram R, Paliany AS, Ho GF, Wan Ishak WZ, Johari CS, et al. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: a randomized controlled trial. Asia-Pacific J Clin Oncol. 2018;14(3):179–91. https://doi.org/10.1111/ajco.12758.
Mego M, Chovanec J, Vochyanova-Andrezalova I, Konkolovsky P, Mikulova M, Reckova M, et al. Prevention of irinotecan induced diarrhea by probiotics: a randomized double blind, placebo controlled pilot study. Complement Ther Med. 2015;23(3):356–62. https://doi.org/10.1016/j.ctim.2015.03.008.
Tan CK, Said S, Rajandram R, Wang Z, Roslani AC, Chin KF. Pre-surgical Administration of microbial cell preparation in colorectal cancer patients: a randomized controlled trial. World J Surg. 2016;40(8):1985–92. https://doi.org/10.1007/s00268-016-3499-9.
Kotzampassi K, Stavrou G, Damoraki G, Georgitsi M, Basdanis G, Tsaousi G, et al. A four-probiotics regimen reduces postoperative complications after colorectal surgery: a randomized, double-blind, placebo-controlled study. World J Surg. 2015;39(11):2776–83. https://doi.org/10.1007/s00268-015-3071-z.
Liu Z, Li C, Huang M, Tong C, Zhang X, Wang L, et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: a double-center and double-blind randomized clinical trial. BMC Gastroenterol. 2015;15:34. https://doi.org/10.1186/s12876-015-0260-z.
Liu ZH, Huang MJ, Zhang XW, Wang L, Huang NQ, Peng H, et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double-center and double-blind randomized clinical trial. Am J Clin Nutr. 2013;97(1):117–26. https://doi.org/10.3945/ajcn.112.040949.
Zhang JW, Du P, Gao J, Yang BR, Fang WJ, Ying CM. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am J Med Sci. 2012;343(3):199–205. https://doi.org/10.1097/MAJ.0b013e31823aace6.
Sadahiro S, Suzuki T, Tanaka A, Okada K, Kamata H, Ozaki T, et al. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: prospective randomized trial. Surgery. 2014;155(3):493–503. https://doi.org/10.1016/j.surg.2013.06.002.
Lee JY, Chu SH, Jeon JY, Lee MK, Park JH, Lee DC, et al. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Dig Liver Dis. 2014;46(12):1126–32. https://doi.org/10.1016/j.dld.2014.09.004.
Brown DG, Borresen EC, Brown RJ, Ryan EP. Heat-stabilised rice bran consumption by colorectal cancer survivors modulates stool metabolite profiles and metabolic networks: a randomised controlled trial. Br J Nutr. 2017;117(9):1244–56. https://doi.org/10.1017/s0007114517001106.
Sheflin AM, Borresen EC, Kirkwood JS, Boot CM, Whitney AK, Lu S et al. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors. Mol Nutr Food Res. 2017;61(1). doi:https://doi.org/10.1002/mnfr.201500905.
Mathers JC, Movahedi M, Macrae F, Mecklin J-P, Moeslein G, Olschwang S, et al. Long-term effect of resistant starch on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. The Lancet Oncology. 2012;13(12):1242–9. https://doi.org/10.1016/S1470-2045(12)70475-8.
Malcomson FC, Willis ND, McCallum I, Xie L, Lagerwaard B, Kelly S, et al. Non-digestible carbohydrates supplementation increases miR-32 expression in the healthy human colorectal epithelium: a randomized controlled trial. Mol Carcinog. 2017;56(9):2104–11. https://doi.org/10.1002/mc.22666.
Malcomson FC, Willis ND, McCallum I, Xie L, Ibero-Baraibar I, Leung WC, et al. Effects of supplementation with nondigestible carbohydrates on fecal calprotectin and on epigenetic regulation of SFRP1 expression in the large-bowel mucosa of healthy individuals. Am J Clin Nutr. 2017;105(2):400–10. https://doi.org/10.3945/ajcn.116.135657.
Eid N, Osmanova H, Natchez C, Walton G, Costabile A, Gibson G, et al. Impact of palm date consumption on microbiota growth and large intestinal health: a randomised, controlled, cross-over, human intervention study. Br J Nutr. 2015;114(8):1226–36. https://doi.org/10.1017/s0007114515002780.
Le Leu RK, Winter JM, Christophersen CT, Young GP, Humphreys KJ, Hu Y, et al. Butyrylated starch intake can prevent red meat-induced O6-methyl-2-deoxyguanosine adducts in human rectal tissue: a randomised clinical trial. Br J Nutr. 2015;114(2):220–30. https://doi.org/10.1017/s0007114515001750.
Windey K, De Preter V, Huys G, Broekaert WF, Delcour JA, Louat T, et al. Wheat bran extract alters colonic fermentation and microbial composition, but does not affect faecal water toxicity: a randomised controlled trial in healthy subjects. Br J Nutr. 2015;113(2):225–38. https://doi.org/10.1017/s0007114514003523.
Humphreys KJ, Conlon MA, Young GP, Topping DL, Hu Y, Winter JM, et al. Dietary manipulation of oncogenic microRNA expression in human rectal mucosa: a randomized trial. Cancer Prev Res (Philadelphia, Pa). 2014;7(8):786–95. https://doi.org/10.1158/1940-6207.capr-14-0053.
Fechner A, Fenske K, Jahreis G. Effects of legume kernel fibres and citrus fibre on putative risk factors for colorectal cancer: a randomised, double-blind, crossover human intervention trial. Nutr J. 2013;12:101. https://doi.org/10.1186/1475-2891-12-101.
Flesch AT, Tonial ST, Contu PC, Damin DC. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: a randomized, double-blind clinical trial. Revista do Colegio Brasileiro de Cirurgioes. 2017;44(6):567–73. https://doi.org/10.1590/0100-69912017006004.
Theodoropoulos GE, Memos NA, Peitsidou K, Karantanos T, Spyropoulos BG, Zografos G. Synbiotics and gastrointestinal function-related quality of life after elective colorectal cancer resection. Ann Gastroenterol. 2016;29(1):56–62.
Krebs B. Prebiotic and Synbiotic treatment before colorectal surgery--randomised double blind trial. Collegium antropologicum. 2016;40(1):35–40.
Wu XD, Xu W, Liu MM, Hu KJ, Sun YY, Yang XF, et al. Efficacy of prophylactic probiotics in combination with antibiotics versus antibiotics alone for colorectal surgery: a meta-analysis of randomized controlled trials. J Surg Oncol. 2018;117(7):1394–404. https://doi.org/10.1002/jso.25038.
Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatology. 2015;12:303–10. https://doi.org/10.1038/nrgastro.2015.47.
Krumbeck JA, Walter J, Hutkins RW. Synbiotics for improved human health: recent developments, challenges, and opportunities. Annu Rev Food Sci Technol. 2018;9:451–79. https://doi.org/10.1146/annurev-food-030117-012757.
He D, Wang HY, Feng JY, Zhang MM, Zhou Y, Wu XT. Use of pro−/synbiotics as prophylaxis in patients undergoing colorectal resection for cancer: a meta-analysis of randomized controlled trials. Clinics and research in hepatology and gastroenterology. 2013;37(4):406–15. https://doi.org/10.1016/j.clinre.2012.10.007.
Funding
C.M.P. is supported by a Canadian Institutes of Health Research (CIHR) New Investigator Salary Award and the Campus Alberta Innovates Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This Article is part of the Topical Collection on Nutrition and Nutritional Interventions in Colorectal Cancer
Rights and permissions
About this article
Cite this article
Mota, J.F., Walter, J. & Prado, C.M. Insights Into the Relationship Between Gut Microbiota and Colorectal Cancer. Curr Colorectal Cancer Rep 14, 251–265 (2018). https://doi.org/10.1007/s11888-018-0419-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-018-0419-4