Abstract
Citronella candles are widely used as insect repellants, especially outdoors in the evening. Because these essential oils are unsaturated, they have a unique potential to form secondary organic aerosol (SOA) via reaction with ozone, which is also commonly elevated on summer evenings when the candles are often in use. We investigated this process, along with primary aerosol emissions, by briefly placing a citronella tealight candle in a smog chamber and then adding ozone to the chamber. In repeated experiments, we observed rapid and substantial SOA formation after ozone addition; this process must therefore be considered when assessing the risks and benefits of using citronella candle to repel insects.
Similar content being viewed by others
Introduction
Exposure to fine particulate matter (aerosol) poses a significant health concern by increasing mortality and morbidity (Peng et al. 2005). Organic aerosol comprises 20 − 90% of the total fine-particle mass, but there are large uncertainties regarding the sources, atmospheric chemistry, and fate of organic aerosol (Hallquist et al. 2009a). One of the biggest sources is incomplete combustion, including both fossil-fuel burning in internal combustion engines and biomass burning at many scales, ranging from enclosed stoves to wild fires (Seinfeld and Pankow 2003).
The scope of this work is to consider emissions by the combustion of citronella oil candles, which are used to ward off mosquitoes. We seek to quantify the emissions of primary organic aerosol (POA) from candle combustion as well as the production of secondary organic aerosol (SOA) from the oxidation of vapors also emitted during combustion. The ingredients specific to citronella candles are mostly unsaturated terpenes; these include limonene and geraniol, as well as a host of minor constituents (Ritter 2006). These candles are used outside on summer evenings, so the oxidation to form SOA by ozone is thus of special interest. The goal of this work is to quantify the SOA in terms of the amount and the timescale for the formation. We carried out experiments in a large smog chamber with atmospherically relevant organic aerosol concentrations, with only a few seconds of candle emissions filling the chamber with ~10 μg m − 3 of aerosol mass. We oxidized the accompanying vapors with ozone to observe any subsequent SOA formation, which, on a warm summer evening, could substantially alter the exposure pattern of individuals in the candle plume.
Background
Combustion is most frequently viewed as a POA source, but recent work has shown that emission of low-volatility vapors from combustion may be a more significant organic aerosol source due to subsequent SOA formation from these vapors (Robinson et al. 2007; Weitkamp et al. 2008; Grieshop et al. 2009a; Donahue et al. 2009). A key aspect of that work was to realize that primary sources must be diluted to ambient conditions (organic aerosol concentrations) for the organic phase partitioning to reflect the atmosphere (Donahue et al. 2006); fresh particles can lose up to 90% of their mass as material evaporates while they are diluted from near-source to near-ambient conditions (Grieshop et al. 2009b). Those vapors, and other vapors co-emitted with the particles, can be oxidized to form large quantities of SOA, which arises from the semi-volatile partitioning of low-volatility reaction products (Odum et al. 1996; Donahue et al. 2006). One important oxidation pathway to form SOA is ozonolysis. Important biogenic species, like α-pinine (Odum et al. 1996; Presto and Donahue 2006) or limonene (Leungsakul et al. 2005; Zhang et al. 2006) are known as a source for a high potential SOA formation through the reaction with ozone because they are unsaturated and cyclic.
Organics are important constituent of aerosols and thus a health concern. In their extended Harvard six cities study, Laden et al. (2006) reported that the PM2.5 concentration is directly linked to a higher cardiovascular and lung cancer mortality. A similar study was conducted by Hoek et al. (2002) in the Netherlands to determine the relationship between traffic- related air pollution and mortality. They concluded that emissions from motor vehicles lead to elevated death rates. Seasonal and spatial patterns of dose-response functions for mortality and PM also suggest that PM composition plays a role in the health effects (Peng et al. 2005), but a direct link between PM composition and health outcomes remains elusive.
The reason we are focusing on aerosols associated with citronella candles is because the candles are used to protect health (and avoid nuisance) by warding off mosquitos. Insect borne diseases are a major source of illness. According to the World Health Organization, almost one million people are killed by malaria every year (WHO 2004). Consequently, insect repellants are an important public health tool. Typically, they are either synthetically produced compounds or plant-derived essential oils. One commonly used chemical repellent is N,N-diethyl-3-methylbenzamide (DEET), while citronella oil is as an example of a plant-derived repellent (Novak and Gerberg 2005).
DEET is used for public health treatment, emergencies and military operations (Novak and Gerberg 2005). It has been the subject of substantial toxicological scrutiny due to some observations of health problems after DEET application (Antwi et al. 2008). Antwi et al. (2008) concluded there are no significant toxicological risks to use DEET as an insect repellent, assumed that it is properly applied. Fradin and Day (2002) also concluded in their study that products containing DEET have a remarkable safety profile, and also determined that DEET products have a better combination of safety and long-lasting effect than non-DEET products.
However, citronella oil is often seen as a safe, natural alternative to DEET, (Ritter 2006) and studies have shown that it is effective if applied in sufficient quantities (Fradin and Day 2002). Citronella oil is obtained from citronella grass. Insect repellants are formulated from extracts of Lenabatu grass, and comprise a complex mixture of hydrocarbons containing terpenes, aldehydes and alcohols. Terpenes dominate. According to the analysis by Wijesekera (1973), the dominant constituents in the Lenabatu-type extract are geraniol and limonene.
The ubiquity of unsaturated compounds in citronella oil is significant because the oil is used in candles burned outside on summer evenings, (Fradin and Day 2002, Sudakin and Trevathan 2003) with the very purpose being dispersal of the active ingredients in the candle plume. Two important aspects about the candle burning should be noted in this context. First, the combustion is not completely efficient, and so many vapors escape the flame front and get dispersed in the candle plume: this is the desired effect for a citronella candle, as the essential oils are thus dispersed. Second, partial combustion can occur in the flame, and consequently compounds can be emitted from the flame that were not present in the original candle formulation.
Consequently, one aspect of using citronella candle that has heretofore been ignored is that the candle emissions may react with ambient ozone (which is often at high concentrations in summer evenings). This reaction may produce SOA, and exposure to that SOA would then be an additional consideration when assessing the relative risks of various insect repelling strategies. Furthermore, because the candles are used expressly for their health benefits, and because the POA emissions will exhibit volatility, this system is an excellent test case to introduce the health-effects community to our new framework for treating the coupled partitioning and chemical aging of organic aerosol (Donahue et al. 2006).
Because of this, we set out to experimentally determine the emissions of POA and the potential formation of SOA from citronella candles, using a smog chamber under conditions as close as possible to ambient organic aerosol levels in order to promote natural phase partitioning. Our major objective was to test whether substantial quantities of SOA could be formed when citronella candle vapors were exposed to ozone.
Experimental methods
We conducted experiments in the Carnegie Mellon University Center for Atmospheric Particle Studies smog-chamber, which has been described extensively in the literature (Presto et al. 2005; Huff Hartz et al. 2005; Grieshop et al. 2009a). The chamber is a 10 m3 flexible Teflon bag (Welch Fluorocarbon) suspended in a temperature-controlled room (15–40 °C) held at 20–22 °C (RH ~10%) for these experiments. Previous experiments have shown modest to no RH dependence in SOA mass yields in chambers (Hallquist et al. 2009b; Prisle et al. 2010).
Particle size distributions were measured with a Scanning Mobility Particle Sizer (SMPS, TSI 3936) operating at a 10:1 flow ratio (typically 5 lpm for sheath flow and 0.5 lpm for aerosol given the small particle sizes observed). Ozone was measured via UV absorption at 2,537 Å (Dasibi 1008-PC). Organic vapors were measured with a quadruple Proton Transfer Reaction - Mass Spectrometer (PTR-MS, Ionicon Analytik GmbH). We measured selected ions in the PTR-MS at m/z = 21, 25, 32, 37 as well as m/z = 81, 137 for terpenes (Hewitt et al. 2003; Zhang et al. 2006) with varying dwell times to optimize signal to noise. Additional masses for protonated citronella oil constituents were also monitored, but m/z = 81 proved to be the most useful for these experiments.
Before each experiment the bag was cleaned overnight at elevated temperature with an ozone purge under UV illumination (360 nm black lights), then filled with particle-free filtered air. We used commercial (Coghland) citronella oil tea-light candles for these experiments. The manufacturer verified that they contained the expected complex mixture of citronella oil. A candle was lighted in advance to make sure the combustion was in a stationary burning phase, without smoldering, before being sampled. The lighted candle was placed in the bag via a sealable opening, which was closed afterwards.
A total of eight experiments were conducted with the candle held in the chamber for varying durations (10, 30, 60, 120 s). To improve the dispersion of the candle plume, an air line was opened for approximately 5 min after the injection to “top off” the chamber, after which ten cycles were sampled with the PTR-MS to obtain a stable signal for the precursors. Because of the very large chamber volume (initially 10 m3), neither the combustion nor inflow during either combustion or the 5-min “top off” period significantly affected bulk parameters (RH, T, etc.). After this, ozone was generated using a corona-discharge ozone generator (Azco HTU500AC) and flushed into the chamber, reaching initial concentrations of 1–2 ppm. Each experiment then continued in this batch mode for about 3 h.
Results and discussion
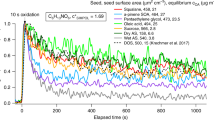
We weighed candles before and after a fixed-length burn to obtain a burning rate of 0.51 mg s − 1. The PTR-MS signal at m/z = 81 (characteristic of terpenes) was proportional to the injection time, as shown for three experiments in Fig. 1 using standard calibration factors for monoterpenes (Zhang et al. 2006). The primary aerosol levels were low, variable, and in some cases negligible, with an emission factor ranging from 4.3–204 μg g − 1. Figure 2 shows a typical primary size distribution with about 2,000 particles cm − 3 peaked at 11 nm. This size range agrees with other publications concerning candle burning. Fine et al. (1999) concluded that particles for candles during normal burning (no sooting or smoldering) are smaller than 100 nm, while Wright et al. (2007) determined that aerosols from paraffin wax tealight candles are in the range of 0.4 nm–11 μm.
Size distribution for POA and SOA. The y axis on the left side refers to the POA 14 min after the candle input whereas the y axis on the right side refers to SOA 7 min after the ozone input. Particle number increases by almost one order of magnitude after ozone injection and the mean diameter also increases significantly, indicating very substantial secondary organic aerosol formation
A crucial issue is that the emissions were heavily diluted. With a candle burn of order 100 s, about 50 mg of candle was burned, for a total emission of approximately 5 mg m − 3 in our 10 m3 chamber. The organic aerosol mass concentrations were at roughly ambient levels of a few μg m − 3. Many of the primary compounds in the candle formulation have a low but still significant vapor pressure: upon extreme dilution typical of ambient conditions, these compounds, with saturation vapor pressures greater than approximately 10 − 7 torr (Donahue et al. 2009), can and will evaporate from ultrafine particles. For example, C25 alkanes (pentacosane) have a saturation concentration (C *) of about 1 μg m − 3, meaning that for organic aerosol concentrations of about 1 μg m − 3, 50% of the pentacosane will be found in the gas phase. The large majority of paraffins in wax candles are lighter than C25 (Crause and Nieuwoudt 2000), so we would expect substantial vaporization of any unburned emissions after dilution of the candle plume into our chamber.
We have observed this dilution effect for all “primary aerosol” sources we have examined, including diesel emissions (Lipsky and Robinson 2006) and wood burning (Grieshop et al. 2009a), and it is an important, general conclusion. Most of the organic material traditionally classified as POA evaporates quickly after emission, and the resulting vapors are subject to rapid gas-phase oxidation. This oxidation can return a substantial amount of organic carbon to the condensed phase, but in an oxidized state and on different particles from the primary emissions (Robinson et al. 2007; Donahue et al. 2009; Jimenez et al. 2009).
We anticipated that a number of the vapors associated with the citronella candles would be unsaturated, and the PTRMS signals at m/z=81 confirmed this. Consequently, we followed each burn with an injection of ozone. We use elevated ozone concentrations to separate chemical timescales (Pathak et al. 2008) in the experiment from any particle wall losses, which have a timescale of a few hours (Pierce et al. 2008). Terpene chemistry depends on the ozone-alkene stoichiometry because many terpenes are multiply unsaturated, and different double bonds can have very different rate constants. For example, limonene has an endo and exo double bond, and the endo double bond reacts almost 30 times more rapidly with ozone (Zhang et al. 2006). Consequently, the reaction products change dramatically as one moves from excess limonene to excess ozone conditions (Sawar and Corsi 2007; Maksymiuk et al. 2009). We operated in excess ozone conditions, which are typical outdoors.
Ozone-alkene reactions generate OH radicals (Atkinson et al. 1992; Paulson et al. 1996; Donahue et al. 1998; Presto and Donahue 2004). Radical scavengers are often employed to isolate ozone-alkene reactions in SOA formation experiments (Keywood et al. 2004), but we elected to let the candle emissions to form their own reactive mixture. Any OH radicals formed via initial ozonolysis will thus have reacted very rapidly with other candle vapors (either saturated or unsaturated, depending on OH rate constants and species abundance); the rate-limiting step in all cases was the initial ozone-alkene reaction. Our objective was not to exactly quantify an SOA formation efficiency from these candles, which in any event turned out to be highly variable, but rather to confirm that SOA formation would be very significant, overwhelming POA emissions even though candles are generally regarded as sources of primary particles.
As we hypothesized, after ozone injection both the particle size and number grew rapidly, indicating new particle formation followed by condensational growth of SOA. For example, in Fig. 2 it can be seen that the particle mode shifted from 11 to 21 nm after 7 min of exposure to ozone, while the total number (the area under each curve) increased from 2,000 to 420,000 cm − 3. The particles continued to grow via SOA condensation for approximately 30 min as the detectible organic precursors vanished along with ozone at rough stoichiometric equivalence.
To determine SOA yields from these experiments we corrected for particle wall losses (Pathak et al. 2008; Pierce et al. 2008) We measured the first-order mass concentration losses at the end of each experiment, when particle growth was negligible; wall loss rate constants were in the range of 0.21–0.38 h − 1. Because the chemical timescale was much shorter than this, the SOA yields were only slightly sensitive to the wall-loss rate constant. Figure 3 shows the wall-loss corrected SOA mass (assuming a density of 1 g cm − 3 and spherical particles) and the signal at m/z = 81 for an experiment resulting in over 30 μg m − 3 of SOA. This was at the very low end of typical yields; in many cases we observed several hundred μg m − 3 of SOA within a few minutes. We report “normalized” SOA mass yields assuming ρ = 1 g cm − 3 when measuring SMPS volume only (Pathak et al. 2008); here any uncertainty in density is dwarfed by the variability in yields from run to run. Overall, the mass yield of SOA relative to the mass of signal at m/z = 81 ranged between 2% and 20%. This is not simply a partitioning effect (Donahue et al. 2006) because experiments with similar apparent precursor consumption showed very different mass yields.
Precursor loss and SOA formation in a typical experiment (t candle = 120 s). Time t=0 refers to ozone input. The y axis on the left side refers to the precursor m/z = 81 signal on the PTR-MS whereas the y axis on the right side refers to mass concentration SOA derived from SMPS measurements. Rapid SOA formation is clearly evident
In spite of the tight correlation between ROG production and candle-burning time shown in Fig. 1, the SOA concentrations showed no clear correlation with burning time. Therefore, we conclude that the amount of SOA formation is dependent on what kind of constituents escape the flame and are dispersed as vapors. It is also possible that some secondary products are formed in the flame, which in turn react with ozone rapidly to form the SOA: yields of these products could be quite variable depending on flame conditions. The signal at m/z = 81 thus appears to be a general but not specific tracer for SOA precursors. The exact precursors responsible for SOA formation (and typical constituents in the SOA) will require more analytical work on the candles, emitted vapors, and SOA products.
Since the citronella oil contains abundant terpenes, particularly monoterpenes like geraniol and limonene, it is likely that these are contributors to the SOA formation via reaction with ozone. For example, the reaction between limonene and ozone as a strong source for SOA (Zhang et al. 2006; Sawar and Corsi 2007). However, we do not know the exact identity of the SOA precursors in these experiments, so we would like to determine the overall rate constant as an additional constraint. The decline in m/z = 81 was very rapid, as was a concurrent decline in ozone (ozone was in excess), and the overall time evolution of reactants and products was consistent with a pseudo first-order system rate-limited by the reaction of ozone with the measured precursors. A pseudo first-order analysis indicates an average rate constant of k ≃ 2 ×10 − 15 cm3 molecule − 1 s − 1. This is significantly higher than the known ozonolysis rate constants for citronella oil constintuents, including limonene (Zhang et al. 2006) and geraniol (Forester et al. 2007). It is more consistent with reactions involving ozone and sesquiterpenes (Calvert et al. 2000). Therefore, we conclude that the observed SOA arises from either relatively minor but highly reactive constituents of the citronella oil or else reactive compounds formed with the flame itself from citronella oil precursors.
It may be convenient to consider an “SOA emission factor”, though this is a gross oversimplification of the formation of semi-volatile constituents (Odum et al. 1996; Donahue et al. 2006). However, this can still be useful to compare POA and SOA production. Based on our experiments, we find an SOA emission rate of 3,300–11,500 μg g − 1. In general the SOA formation exceeds POA emissions from the candle (4.3–204 μg g − 1) by several orders of magnitude.
POA emission factors in the literature for other candles are somewhat larger than what we observe for citronella candles. Fine et al. (1999) conducted experiments with paraffin and beeswax candles. The emission factors vary between 520–3,720 μg g − 1 for paraffin with slightly higher values for beeswax (1,060–2,040 μg g − 1). They concluded that for unscented candles the POA emission factor is 870±600 μg g − 1. In addition, the burning rates for paraffin are in the range of 75–119 mg min − 1 and 53–63 mg min − 1 for beeswax candles, while the burning rates for citronella oil candles in our experiment are in the range of 30 mg min − 1. Citronella oil candles evidently have a higher specific heat capacity, so that more heat quantity per mass unit is necessary to increase the temperature to a level that the citronella oil wax becomes liquid. Ultimately, the SOA formation we observed from the citronella candles substantially exceeds the observed POA emissions from all candle types.
Conclusions
During the experiments reported here we observed both POA emissions from citronella-oil candles and SOA formation after candle vapors were exposed to ozone. We also observed rapid loss of vapors using a PTR/MS at m/z = 81. However, we did not observe a simple dependance between SOA formation and candle input time. The observed reaction is too rapid for a typical reaction between monoterpenes and ozone, suggesting that more reactive vapors emitted by the burning candle may be important SOA precursors. Future work to measure the composition of vapors emitted by these burning candles would be of great interest.
A unique feature of the citronella candle is the significant emission of unsaturated vapors. These vapors in turn lead to very large SOA formation yields, which overwhelm the POA emissions. It is possible that the same outcome could follow from paraffin candles, only with OH radicals driving the oxidation: we have observed this for other POA sources (Robinson et al. 2007; Grieshop et al. 2009a). However, SOA formation in that case would occur only outdoors during daylight (not a common time for candle burning) and is in any event beyond the scope of this work. The combination of unsaturated vapors from the citronella candles and availability of ozone as an oxidant outdoors in the evening may place these candles in a unique position as SOA precursors leading to localized human exposure during evening socializing outdoors.
We make no claim that the SOA from citronella candles necessarily renders them hazardous. However, any assessment of human exposure must consider the processes between emission and exposure, and in this case atmospheric oxidation is both rapid and consequential. It is very important to consider atmospheric processing when treating health effects from an emissions source, even one that may be quite close to an effected person as in this example. This serves as a vivid illustration that organic aerosols are complex and dynamic. They combine rapid chemical evolution with phase partitioning in ways that are significant both to the chemical constituents individuals are exposed to and to the dosing mechanism (as compounds move between the vapor and condensed phases). These are effects that cannot be ignored in exposure studies.
References
Antwi F, Shama L, Peterson R (2008) Risk assessment for the insect repellents DEET and picaridin. Regul Toxicol Pharmacol 51:31–36
Atkinson R, Aschmann S, Arey J, Shores B (1992) Formation of OH radicals in the gas phase reactions of O3 with a series of terpenes. J Geophys Res 97:6065–6073
Calvert J, Atkinson R, Kerr J, Madronich S, Moortgat G, Wallington T, Yarwood G (2000) The mechanisms of atmospheric oxidation of the alkenes. Oxford University Press, New York
Crause JC, Nieuwoudt I (2000) Fractionation of paraffin wax mixtures. Ind Eng Chem Res 39:4871–4876
Donahue N, Robinson A, Pandis N (2009) Atmospheric organic particulate matter from smoke to secondary organic aerosol. Atmos Environ 43:94–106
Donahue N, Robinson A, Stanier C, Pandis N (2006) Coupled partitioning, dilution, and chemical aging of semivolatile organics. Environ Sci Technol 40:2635–2643
Donahue NM, Kroll JH, Anderson JG, Demerjian KL (1998) Direct observation of OH production from the ozonolysis of olefins. Geophys Res Lett 25:59
Fine P, Cass G, Simoneit B (1999) Characterization of fine particle emissions from burning church candles. Environ Sci Technol 33:2352–2362
Forester C, Ham J, Wells J (2007) Geraniol (2,6-dimethyl-2,6-octadien-8-ol) reactions with ozone and oh radical: rate constants and gas-phase products. Atmos Environ 41:1188–1199
Fradin M, Day J (2002) Comparative efficiency of insect repellents against mosquitos bites. N Engl J Med 347(1):13–18
Grieshop A, Donahue N, Robinson A (2009a) Laboratory investigation of photochemical oxidation of organic aerosol from wood fires 1 measurement and simulation of organic aerosol. Atmos Chem Phys 9:1263–1277
Grieshop AP, Miracolo MA, Donahue NM, Robinson AL (2009b) Constraining the volatility distribution and gas-particle partitioning of combustion aerosols using isothermal dilution and thermodenuder measurements. Environ Sci Technol 43:4750–4756 doi:10.1021/es8032378. http://pubs.acs.org/doi/abs/10.1021/es8032378
Hallquist M, Wenger J, Baltensperger U (2009a) The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos Chem Phys Discuss 9:3555–3762
Hallquist M, Wenger JC, Baltensperger U, Rudich Y, Simpson D, Claeys M, Dommen J, Donahue NM, George C, Goldstein AH, Hamilton JF, Herrmann H, Hoffmann T, Iinuma Y, Jang M, Jenkin ME, Jimenez JL, Kiendler-Scharr A, Maenhaut W, McFiggans G, Mentel TF, Monod A, Prévôt ASH, Seinfeld JH, Surratt JD, Szmigielski R, Wildt J (2009b) The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos Chem Phys 10:5155–5236. http://www.atmos-chem-phys.net/9/5155/2009/
Hewitt C, Hayward S, Tani A (2003) The application of proton transfer reaction-mass spectrometry (ptr-ms) to the monitoring and analysis of volatile organic compounds in the atmosphere. J Environ Monit 5:1–7
Hoek G, Brunekreef B, Goldbohm S, Fischer P, van den Brandt PA (2002) Association between mortality and indicators of traffic-related air pollution in the netherlands: a cohort study. Lancet 360(9341):1203–1209. Times Cited: 391
Huff Hartz KE, Rosenørn T, Ferchak SR, Raymond TM, Bilde M, Donahue NM, Pandis SN (2005) Cloud condensation nuclei activation of monoterpene and sesquiterpene secondary organic aerosol. J Geophys Res 110:D14,208. http://www.agu.org/pubs/crossref/2005.../2004JD005754.shtml
Jimenez JL, Canagaratna MR, Donahue NM, Prévôt, ASH, Zhang Q, Kroll JH, DeCarlo PF, Allan J, Coe H, Ng NL, Aiken AC, Docherty KD, Ulbrich IM, Grieshop AP, Robinson AL, Duplissy J, Smith JD, Wilson KR, Lanz VA, Hueglin C, Sun YL, Laaksonen A, Raatikainen T, Rautiainen J, Vaattovaara P, Ehn M, Kulmala M, Tomlinson JM, Collins DR, Cubison MJ, Dunlea EJ, Huffman JA, Onasch TB, Alfarra MR, Williams PI, Bower K, Kondo Y, Schneider J, Drewnick F, Borrmann S, Weimer S, Demerjian K, Salcedo D, Cottrell L, Griffin R, Takami A, Miyoshi T, Hatakeyama S, Shimono A, Sun JY, Zhang YM, Dzepina K, Kimmel JR, Sueper D, Jayne JT, Herndon SC, Trimborn AM, Williams LR, Wood EC, Kolb CE, Baltensperger U, Worsnop DR (2009) Evolution of organic aerosols in the atmosphere: a new framework connecting measurements to models. Science 326:1525–1529
Keywood MD, Kroll JH, Varutbangkul V, Bahreini R, Flagan RC, Seinfeld JH (2004) Secondary organic aerosol formation from cyclohexene ozonolysis: effect of OH scavenger and the role of radical chemistry. Environ Sci Technol 38:3343
Laden F, Schwartz J, Speizer F, Dockery D (2006) Reduction in fine particulate air pollution and mortality extended follow-up of the harvard six cities study. Am J Respir Crit Care Med 173:667–672
Leungsakul S, Jaoui M, Kamens RM (2005) Kinetic mechanism for predicting secondary organic aerosol formation from the reaction of d-limonene with ozone. Environ Sci Technol 39:9583
Lipsky EM, Robinson AL (2006) Effects of dilution on fine particle mass and partitioning of semivolatile organics in diesel exhaust and wood smoke. Environ Sci Technol 40:155–162
Maksymiuk CS, Gayathri C, Gil RR, Donahue NM (2009) Secondary organic aerosol formation from multiphase oxidation of limonene by ozone: mechanistic constraints via two-dimensional heteronuclear NMR spectroscopy. Phys Chem Chem Phys 11:7810–7818 doi:10.1039/b820005j. http://www.rsc.org/Publishing/Journals/CP/article.asp?doi=b820005j
Novak R, Gerberg E (2005) Natural-based repellent products. efficacy for military and general publc uses. J Am Mosq Control Assoc 21(4):7–11
Odum J, Hoffmann T, Bowman F, Collins D, Flagan R, Seinfeld J (1996) Gas/particle partitioning and secondary organic aerosol yields. Environ Sci Technol 30:2580
Odum JR, Hoffmann T, Bowman FA, Collins D, Flagan RC, Seinfeld JH (1996) Gas/particle partitioning and secondary organic aerosol yields. Environ Sci Technol 30:2580
Pathak RK, Huff Hartz KE, Donahue NM, Pandis SN (2008) Ozonolysis of β-pinene: temperature dependence of secondary organic aerosol mass fraction. Environ Sci Technol 42:5081–5086. doi:10.1021/es070721z. http://pubs.acs.org/cgi-bin/abstract.cgi/esthag/2008/42/i14/abs/es070721z.html
Paulson SE, Orlando JJ (1996) The reactions of ozone with alkenes: An important source of HOx in the boundary layer. Geophys Res Lett 23(25):3727–3730
Peng R, Dominici F, Pastor-Barriuso R, Zeger S, Samet J (2005) Seasonal analyses of air pollution and mortality in 100 US cities. Am J Epidemiol 161(6):585–594. doi:10.1093/aje/kwi075
Pierce J, Engelhart G, Hildebrandt L, Weitkamp E, Pathak R, Donahue NM, Robinson A, Adams P, Pandis N (2008) Constraining particle evolution from wall losses, coagulation, and condensation-evaporation in smog-chamber experiments: optimal estimation based on size distribution measurements. Aerosol Sci Tech 42:1001–1015
Presto A, Donahue N (2006) Investigation of apinene and ozone secondary organic aerosol formation at low total aerosol mass. Environ Sci Technol 40:3536–3543
Presto AA, Donahue NM (2004) Ozonolysis fragment quenching by nitrate formation: the pressure dependence of prompt OH radical formation. J Phys Chem, A 108:9096–9104. http://pubs.acs.org/doi/abs/10.1021/jp047162s
Presto AA, Hartz KEH, Donahue NM (2005) Secondary organic aerosol production from terpene ozonolysis. 1. Effect of UV radiation. Environ Sci Technol 39(18):7036–7045. Times Cited: 34
Prisle NL, Engelhart GJ, Bilde M, Donahue NM (2010) Humidity effects on organic aerosol partitioning following the α-pinene + ozone reaction. Geophys Res Lett 37(1). doi:10.1029/2009GL041402
Ritter S (2006) What’s that stuff? citronella oil. Chem Eng News: Sci Technol 84(44):42–44
Robinson A, Donahue N, Shrivastava M, Weitkamp E, Sage A, Grieshop A, Lane T, Pierce J, Pandis N (2007) Rethinking organic aerosols semivolatile emissions and photochemical aging. Science 315:1259–1262
Sawar G, Corsi R (2007) The effects of ozone/limonene reactions on indoor secondary organic aerosols. Atmos Environ 41:959–973
Seinfeld J, Pankow J (2003) Organic atmospheric particulate material. Annu Rev Phys Chem 54:121–140
Sudakin D, Trevathan W (2003) Deet a review and update of safety and risk in the general population. J Toxicol 41(6):831–839
Weitkamp E, Lambe A, Donahue NM, Robinson A (2008) Laboratory measurements of the heterogenous oxidation of condensed-phase organic molecular makers for motor vehicle exhaust. Environ Sci Technol 42:7950–7956
WHO (2004) The global burden of disease: 2004 update. World Health Organization
Wijesekera R (1973) The chemical composition and analysis of citronella oil. J Natl Sci Counc Sri Lanka 1:67–81
Wright M, Fews A, Keitch P, Henshaw D (2007) Small-ion and nano-aerosol production during candle burning size and concentration profile with time. Aerosol Sci Tech 41:475–484
Zhang J, Huff Hartz K, Pandis N, Donahue N (2006) Secondary aerosol formation from limonene ozonolysis homogenous and heterogenous influences as a function of NO x . J Phys Chem, A 110:11053–11063
Author information
Authors and Affiliations
Corresponding author
Additional information
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Bothe, M., Donahue, N.M. Organic aerosol formation in citronella candle plumes. Air Qual Atmos Health 3, 131–137 (2010). https://doi.org/10.1007/s11869-009-0061-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-009-0061-z