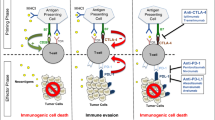
Opinion statement
Checkpoint inhibitors have monumentally transformed the treatment of metastatic urothelial carcinoma. While the efficacy and safety of the different agents are similar in platinum-refractory metastatic urothelial carcinoma, pembrolizumab is the only agent that was superior to chemotherapy in a randomized phase III trial. Pembrolizumab and atezolizumab are also approved as first-line therapies in cisplatin-ineligible metastatic urothelial carcinoma. Several immunotherapy trials are ongoing in non-metastatic setting to maximize responses upfront. Despite the promising responses with immunotherapy, majority of patients do not respond to monotherapy and combination approaches would be the path moving forward to maximize responses. In addition, novel therapies are needed for patients who progress on checkpoint inhibitors. There is still a lot to be done to better understand predictive biomarkers, optimal combination, and sequences to improve clinical outcomes in urothelial carcinoma.
Similar content being viewed by others
Change history
23 May 2019
In the original version of this article, which published in Current Treatment Options in Oncology, Volume 20, Issue 12, December 2018, the surname of the third author was captured incorrectly. The name shown above is correct.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance•• Of major importance
Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180–3.
Brandau S, Suttmann H. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: a success story with room for improvement. Biomed Pharmacother. 2007;61(6):299–305. https://doi.org/10.1016/j.biopha.2007.05.004.
Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–8. https://doi.org/10.1038/nature12213.
Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med. 2016;375(18):1767–78. https://doi.org/10.1056/NEJMra1514296.
Ribas A. Releasing the Brakes on Cancer Immunotherapy. N Engl J Med. 2015;373(16):1490–2. https://doi.org/10.1056/NEJMp1510079.
Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–20. https://doi.org/10.1016/S0140-6736(16)00561-4.
• Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748–57. https://doi.org/10.1016/S0140-6736(17)33297-X. Randomized phase 3 trial, comparing atezolizumab with chemotherapy comparing response rate and survival outcomes.
•• Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376(11):1015–26. https://doi.org/10.1056/NEJMoa1613683. Randomized phase 3 trial, comparing chemotherapy vs pembrolizumab, showing overall survival benefit with pembrolizumab.
Plimack ER, Bellmunt J, Gupta S, Berger R, Chow LQ, Juco J, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017;18(2):212–20. https://doi.org/10.1016/S1470-2045(17)30007-4.
Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17(11):1590–8. https://doi.org/10.1016/S1470-2045(16)30496-X.
Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–22. https://doi.org/10.1016/S1470-2045(17)30065-7.
Powles T, O'Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol. 2017;3(9):e172411. https://doi.org/10.1001/jamaoncol.2017.2411.
Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, et al. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J Clin Oncol. 2017;35(19):2117–24. https://doi.org/10.1200/JCO.2016.71.6795.
Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19(1):51–64. https://doi.org/10.1016/S1470-2045(17)30900-2.
Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, et al. Treatment of patients with metastatic urothelial cancer "unfit" for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29(17):2432–8. https://doi.org/10.1200/JCO.2011.34.8433.
Li J, Juliar B, Yiannoutsos C, Ansari R, Fox E, Fisch MJ, et al. Weekly paclitaxel and gemcitabine in advanced transitional-cell carcinoma of the urothelium: a phase II Hoosier Oncology Group study. J Clin Oncol. 2005;23(6):1185–91. https://doi.org/10.1200/jco.2005.05.089.
De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M, Maroto P, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30(2):191–9. https://doi.org/10.1200/jco.2011.37.3571.
Gitlitz BJ, Baker C, Chapman Y, Allen HJ, Bosserman LD, Patel R, et al. A phase II study of gemcitabine and docetaxel therapy in patients with advanced urothelial carcinoma. Cancer. 2003;98(9):1863–9. https://doi.org/10.1002/cncr.11726.
•• Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–92. https://doi.org/10.1016/S1470-2045(17)30616-2. Phase 2 study showing overall response rate and survival outcomes of pembrolizumab in cisplatin-ineligible patients.
•• Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. https://doi.org/10.1016/S0140-6736(16)32455-2. Phase 2 study showing overall response rate and survival outcomes of atezolizumab in cisplatin-ineligible patients.
Necchi A, Briganti A, Raggi D, Giannatempo P, Mariani L, Messina A et al. Interim results from PURE-01: A phase 2, open-label study of neoadjuvant pembrolizumab (pembro) before radical cystectomy for muscle-invasive urothelial bladder carcinoma (MIUC). J Clin Oncol. 2018;36: 6_Suppl.
Powles T, Rodriguez-Vida A, Duran I, Crabb SJ, Heijden MSVD, Pous AF et al. A phase II study investigating the safety and efficacy of neoadjuvant atezolizumab in muscle invasive bladder cancer (ABACUS). J Clin Oncol. 2018;36:15_Suppl:4506-.
Gupta S, Agarwal N, Konety B, Weight C, Thyagarajan B, Murugan PJ et al. Phase II trial of neoadjuvant nivolumab with cisplatin and gemcitabine in muscle-invasive bladder cancer patients undergoing radical cystectomy. J Clin Oncol. 2018;36:6_Suppl.
Liakou CI, Narayanan S, Ng Tang D, Logothetis CJ, Sharma P. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human bladder cancer. Cancer Immun. 2007;7:10.
Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. 2016;34(26):3119–25. https://doi.org/10.1200/JCO.2016.67.9761.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. https://doi.org/10.1038/nrc3239.
Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14(4):847–56. https://doi.org/10.1158/1535-7163.MCT-14-0983.
Boorjian SA, Sheinin Y, Crispen PL, Farmer SA, Lohse CM, Kuntz SM, et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res: Off J Am Assoc Cancer Res. 2008;14(15):4800–8. https://doi.org/10.1158/1078-0432.CCR-08-0731.
Riaz N, Morris L, Havel JJ, Makarov V, Desrichard A, Chan TA. The role of neoantigens in response to immune checkpoint blockade. Int Immunol. 2016;28(8):411–9. https://doi.org/10.1093/intimm/dxw019.
Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016;165(1):35–44. https://doi.org/10.1016/j.cell.2016.02.065.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–20. https://doi.org/10.1056/NEJMoa1500596.
Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med. 2017;377(25):2500–1. https://doi.org/10.1056/NEJMc1713444.
Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, et al. Pan-cancer Immunogenomic Analyses Reveal Genotype-Immunophenotype Relationships and Predictors of Response to Checkpoint Blockade. Cell Rep. 2017;18(1):248–62. https://doi.org/10.1016/j.celrep.2016.12.019.
McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–9. https://doi.org/10.1126/science.aaf1490.
Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, et al. Evolution of Neoantigen Landscape during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Discov. 2017;7(3):264–76. https://doi.org/10.1158/2159-8290.CD-16-0828.
Luksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A, et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature. 2017;551(7681):517–20. https://doi.org/10.1038/nature24473.
Kim S, Kim HS, Kim E, Lee MG, Shin EC, Paik S, et al. Neopepsee: accurate genome-level prediction of neoantigens by harnessing sequence and amino acid immunogenicity information. Ann Oncol. 2018;29(4):1030–6. https://doi.org/10.1093/annonc/mdy022.
Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther. 2017;16(11):2598–608. https://doi.org/10.1158/1535-7163.MCT-17-0386.
Aggen DH, Drake CG. Biomarkers for immunotherapy in bladder cancer: a moving target. J Immunother Cancer. 2017;5(1):94. https://doi.org/10.1186/s40425-017-0299-1.
Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–22. https://doi.org/10.1038/nature12965.
Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25(2):152–65. https://doi.org/10.1016/j.ccr.2014.01.009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Rohit Jain declares that he has no conflict of interest.
Travis Snyders declares that he has no conflict of interest.
Lakshminarayanan Nandagopal declares that he has no conflict of interest.
Rohan Garje declares that he has no conflict of interest.
Yousef Zakharia has received compensation for participating on advisory boards for Pfizer, Novartis, Roche Diagnostics, Castle Biosciences, Exelixis, Eisai, Johnson & Johnson, and Amgen.
Shilpa Gupta has received research funding (paid to her institution) from Bristol-Myers Squibb; has received compensation for service on advisory boards from Exelixis, Janssen, ARMO, AstraZeneca, Merck, and Genentech; and has received compensation for service on speakers’ bureaus from Exelixis, Janssen, AstraZeneca, and Genentech.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Genitourinary Cancers
Rights and permissions
About this article
Cite this article
Jain, R.K., Snyders, T., Nandgoapal, L. et al. Immunotherapy Advances in Urothelial Carcinoma. Curr. Treat. Options in Oncol. 19, 79 (2018). https://doi.org/10.1007/s11864-018-0598-x
Published:
DOI: https://doi.org/10.1007/s11864-018-0598-x