Opinion Statement
Advanced melanoma, rarely diagnosed at the time of primary melanoma excision but most often occurring later via lymphatic or hematogenous dissemination, is the cause of death for approximately 10,000 people in the USA each year, with the rate of incidence and death increasing yearly. Its causes are multifactorial and depend in large part on solar ultraviolet damage to DNA as well as underlying genetic predisposition. Cutaneous melanoma is the most common, but other subsets of importance are mucosal and uveal primaries, with different biology and treatment considerations. Mutational oncogenic “drivers” may be targeted with chronically administered, oral kinase inhibitors, currently consisting of the mitogen-activated protein kinase (MAPK) inhibitor combinations of BRAF plus MEK-targeted drugs. These agents work quickly to relieve symptoms and induce remissions but generally have limited durations of disease control. Immunotherapies include the immune checkpoint inhibitors that block CTLA4 or PD-1-negative immune signaling as well as interleukin-2, a cytokine that stimulates T lymphocytes and natural killer cells. A combination of CTLA4 plus PD-1 blockade has the highest activity ever reported for metastatic melanoma, at the cost of high autoimmune-like toxicities. However, immunotherapies of this type may provide durable responses and even cure a subset of patients. Thus, these immunotherapeutic agents are recommended as first-line therapy for most patients with advanced melanoma. Patients with rapidly progressive, symptomatic melanoma whose tumor carries a BRAF mutation may benefit more from initial therapy with combined MAPK inhibitors.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2009;59:225–49.
Cancer Facts & Figs. 2015 American Cancer Society (cancer.org)
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54.
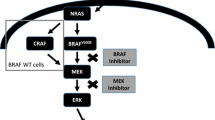
Johnson DB, Menzies AM, Zimmer L, et al. Acquired BRAF inhibitor resistance: a multicenter meta-analysis of the spectrum and frequencies, clinical behaviour, and phenotypic associations of resistance mechanisms. Eur J Cancer. 2015;51:2792–9. Resistance mechanisms to the current only targetable oncogenic driver mutation develop early and often, and increased understanding of their molecular biology and relative representation informs new studies of novel agents to pre-empt or overcome resistance
Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature. 2011;480:387–90.
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16.
Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65.
Hagen B. Managing side effects of vemurafenib therapy for advanced melanoma. J Adv Pract Oncol. 2014;5:400–10. As new drugs with novel mechanism of action present new challenges for toxicity recognition and management, it is important to have references that provide evidence- and experience-based recommendations
Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–14.
Long GV, Stroyakovsky D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–88. The first of several papers to demonstrate improvement in all outcomes for combination vertical MAPK inhibition and to confirm and expand medical knowledge regarding the spectrum of toxicities and side effects
Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–9. Complements the information reported in reference 12
Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–76. The first phase III data and support of the novel combination of vemurafenib and cobimetinib as an alternative to dabrafenib and trametinib
Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32.
Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. First demonstration of nivolumab activity in melanoma and its safety in a randomized trial against chemotherapy for untreated, BRAF wild-type metastatic melanoma
Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7:122–36.
McNeal AS, Liu K, Nakhate V, et al. CDKN2B loss promotes progression from benign melanocytic nevus to melanoma. Cancer Discov. 2015;5:1072–85. A critical molecular explanation for the transition from BRAF v600-mutated benign/senescent nevi to invasive melanoma
Menzies AM, Haydu LE, Visintin I, et al. Distinguishing clinicopathologic features of patients with v600E and v600K mutations. Clin Cancer Res. 2012;18:32–49.
Zhang C, Spevak W, Zhang Y, et al. RAF inhibitors that evade paradoxical MAPK pathway activation. Nature. 2015;526:583–6.
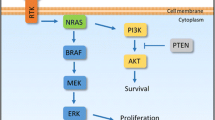
Nissan MH, Pratilas CA, Jones AM, et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res. 2015;74:2340–50 . Discovery of a new driver oncogene with known signaling pathways that accounts for many of the melanomas not dependent on BRAF or NRAS mutation
Renzani M, Alifrangis C, Perna D, et al. BRAF/NRAS wild-type melanoma, NF1 status and sensitivity to trametinib. Pigment Cell Melanoma Res. 2014;28:117–9.
Carvajal RD, Sosman JA, Quevedo JF, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA. 2014;311:2397–405. Proof of principle for the importance of MEK as a druggable target in the biology and therapy of uveal melanoma and confirmation of the extremely low benefit of chemotherapy in uveal melanoma
Cooper ZA, Reuben A, Austin-Breneman J, Wargo JA. Does it MEK a difference? Understanding immune effects of targeted therapy. Clin Cancer Res. 2015;21:3102–4.
Hu-Lieskovan S, Mok S, Homet Moreno B, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med. 2015;7:279ra41.
Niessner H, Forschner A, Klumpp B, et al. Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer Med. 2013;2:76–85.
Bucheit AD, Chen G, Siroy A, et al. Complete loss of PTEN protein expression correlates with shorter time to brain metastasis and survival in stage IIIB/C melanoma patients with BRAFV600 mutations. Clin Cancer Res. 2014;20:5527–36. One of several reports to demonstrate the association of PTEN functional loss with propensity to develop brain metastasis
Carlino MS, Todd JR, Rizos H. Resistance to c-kit inhibitors in melanoma: insights for future therapies. Oncoscience. 2014;1:423–6.
Wu X, Li J, Zhu M, Fletcher JA, Hodi FS. Protein kinase C inhibitor AEB071 targets ocular melanoma harboring GNAQ mutations via effects on the PKC/Erk1/2 and PKC/NF-κB pathways. Mol Cancer Ther. 2012;11:1905–14.
Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–016. Review. The seminal report of a pooled cohort of patients who received high-dose IL-2 for metastatic melanoma and achieved durable remissions (approximately 7 %) and overall response rates (approximately 16–19 %) range, at the cost of substantial but reversible multisystem toxicity
Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–94.
Weber J, Kahler K, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–7.
Vétizou M, Pitt JM, Daillère R. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–84.
Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–9. These two sources are of great importance in demonstrating the critical role of the host microbiome (which can be manipulated) in determining not only the safety of therapy but also therapeutic outcomes
Liang SC, Latchman YE, Buhlmann JE, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–16.
Shin D, Garcia-Diaz A, Zaretsky J, et al. Innate resistance of PD-1 blockade through loss of function mutations in JAK resulting in inability to express PD-L1 upon interferon exposure. J Immunother Cancer. 2015;3(Suppl 2):P311.
Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–18.
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and Ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34.
Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30.
Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol. 2014;9:239–71. Review. A unique review that puts into perspective the molecular biology, etiologic factors and clinical associations in all subsets of melanoma
Shain AH, Garrido M, Botton T, et al. Exome sequencing of desmoplastic melanoma identifies recurrent NFKBIE promoter mutations and diverse activating mutations in the MAPK pathway. Nat Genet. 2015;47:1194–9.
Reardon DA, Gokhale PC, Klein SR et al. (2015) Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol Res
Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. The first clinical report of combination checkpoint blocking antibodies, with the highest reported benefit rate of any therapy for advanced melanoma and a very high rate of immune-related toxicities
Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17.
Teply BA, Lipson EJ. Identification and management of toxicities from immune checkpoint-blocking drugs. Oncology (Williston Park). 2014;28(Suppl 3):30–8. Review
Robert C, Schadendorf D, Messina M, et al. Efficacy and safety of retreatment with ipilimumab in patients with pretreated advanced melanoma who progressed after initially achieving disease control. Clin Cancer Res. 2013;19:2232–9. Retreatment of relapse in previous ipilimumab responders without severe toxicity shows activity, supporting proof of the principle that unlike cytotoxic therapies, immunotherapy relapse does not always result from treatment-resistant tumor cells or immune-tumor interactions
Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–8. Complements #48, in this report using PD-1 blockade
Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–18.
Eggermont AM, Suciu S, Rutkowski P, et al. Long term follow up of the EORTC 18952 trial of adjuvant therapy in resected stage IIB-III cutaneous melanoma patients comparing intermediate doses of interferon-alpha-2b (IFN) with observation: ulceration of primary is key determinant for IFN-sensitivity. Eur J Cancer. 2016;55:111–21.
Eggermont AM, Suciu S, Testori A, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol. 2012;30:3810–28.
Eggermont AM, Suciu S, Testori A, et al. Ulceration and stage are predictive of interferon efficacy in melanoma: results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur J Cancer. 2012;48:218–25.
Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–30.
Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459–65. The first prospective clinical trial of CTLA4 blockade for melanoma metastatic to the brain, showing patient benefit in both the brain and extracranial compartment comparable to that of the same therapy for patients without brain metastasis
Cohen JV, Alomari AK, Vortmeyer AO et al. (2015) Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res
Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579–89.
Ahmed KA, Stallworth DG, Kim Y et al. (2015) Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol
Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84. First demonstration of activity and safety of nivolumab vs. chemotherapy in advanced melanoma patients previously treated with anti-CTLA-4 antibody
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kim Margolin has received honoraria from Bristol-Myers Squibb (BMS) for talks given by herself but sponsored by BMS and has received consulting fees from Roche/Genentech.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Skin Cancer
Rights and permissions
About this article
Cite this article
Margolin, K. The Promise of Molecularly Targeted and Immunotherapy for Advanced Melanoma. Curr. Treat. Options in Oncol. 17, 48 (2016). https://doi.org/10.1007/s11864-016-0421-5
Published:
DOI: https://doi.org/10.1007/s11864-016-0421-5