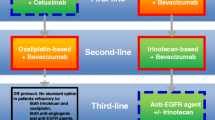
Opinion statement
Metastatic cancer was previously treated with distinctive lines of chemotherapy regimens upon disease progression or toxicity, yet the choices of therapy are actually interrelated, with the selection of a first-line regimen in part determining the choices available for subsequent treatment. Lately the therapeutic approach based on separate lines of treatment, tends to be replaced from a perspective strategical approach, that of the “continuum of care”. This strategy targets to an improved overall survival, improved of quality of life and minimization of toxicity through upfront design of treatment selection and sequencing, exposure to all available drugs and minimization of unnecessary treatment. Anti-VEGF treatment has a well-documented role in this approach. Bevacizumab should be included in upfront treatment regimens for all mCRC patients independently of RAS status, unless contraindicated. Upfront bevacizumab could be combined with all available regimens since the optimal choice of backbone chemotherapy is yet to be defined. In RAS wild-type population, when metastasectomy is the target, an anti-EGFR combination is also a valid approach. Maintenance with bevacizumab and fluoropyrimidines should be considered upon intolerance of induction treatment and/or disease stabilization; maintenance with bevacizumab monotherapy should be avoided. In highly selected patients, complete treatment cessation could be also an option. Continuation with bevacizumab upon first progression and switch of the “backbone” chemotherapy is a validated approach. Patients progressing after first-line oxaliplatin regimen including bevacizumab combinations could be treated with an aflibercept–irinotecan combination. When no more options are available, regorafenib monotherapy should be the following choice. Combinations of anti-VEGF and anti-EGFR treatment have no place in this approach and are not indicated.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Goldberga RM, Rothenbergb ML, et al. The continuum of care: a paradigm for the management of metastatic colorectal cancer. Oncologist. 2007;12(1):38–50.
Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9.
Wolpin BM, Bass AJ. Managing advanced colorectal cancer: have we reached the PEAK with current therapies? J Clin Oncol. 2014;32:2200–2. Interesting commentary on the results of the PEAK trial focusing on the impact of first line choice with anti-EGFR or anti-VEGF combinations for untreated RAS WT patients.
Stintzing S, Jung A, Rossius L, et al. Analysis of KRAS/NRAS and BRAF mutations in FIRE-3: a randomized phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC) patients. ESMO 2013 Abstract LBA17. Final results from the extended RAS testing of the FIRE-3 study resulting in an even more pronounced OS for the RAS WT patients.
Stintzing S, Modest D, Fischer von Weikersthal L, et al. Independent radiological evaluation of objective response rate, early tumor shrinkage, and depth of response in FIRE-3 (AIO KRK-0306) in the final RAS evaluable population. ESMO 2014 Abstract LBA11. Independent post-hoc analysis of the FIRE 3 giving more insight on the benefit of anti-EGFR compared to anti-VEGF combinations in terms of response in treatment naïve RAS WT patients.
Grothey A, Hart LL, Rowland KM, et al. Intermittent oxaliplatin (oxali) administration and time-to-treatment-failure (TTF) in metastatic colorectal cancer (mCRC): final results of the phase III CONcePT trial. J Clin Oncol. 2008;26(suppl 15S):180s.
Díaz-Rubio E, Gómez-España A, Massutí B, et al. First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist. 2012;17(1):15–25.
Koopman M, Simkens LH, Ten Tije AJ, et al. Maintenance treatment with capecitabine and bevacizumab versus observation after induction treatment with chemotherapy and bevacizumab in metastatic colorectal cancer (mCRC): the phase III CAIRO3 study of the Dutch Colorectal Cancer Group (DCCG). J Clin Oncol. 2014;32:5s. Proof for significant benefit for the maintenance with fluoropyrimidines/bevacizumab for PFS in a robust phase III trial.
Koberle D, Betticher DC, Von Moos R, et al. Bevacizumab continuation versus no continuation after first-line chemo-bevacizumab therapy in patients with metastatic colorectal cancer: a randomized phase III non inferiority trial (SAKK 41/06). ASCO 2013; abstr 3503. This trial proved in a non inferiority design a lack of benefit of bevacizumab monotherapy maintenance.
Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360(6):563.
Bekaii-Saab TS, Grothey A, Bendell JC, et al. Effectiveness and safety of second-line (2 L) irinotecan- and oxaliplatin-based regimens after first-line (1 L) bevacizumab (BV)-containing treatment (tx) for metastatic colorectal cancer (mCRC): results from the ARIES observational cohort study (abstract). J Clin Oncol. 2012; 30 (suppl 4): abstract 535.
Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11(1):38–47.
Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GER-COR study. J Clin Oncol. 2004;22:229–37.
Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–14.
Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol. 2005;23:9441–2.
Falcone A, Masi G, Brunetti I, et al. The triplet combination of irinotecan, oxaliplatin and 5FU/LV (FOLFOXIRI) vs the doublet of irinotecan and 5FU/LV (FOLFIRI) as first-line treatment of metastatic colorectal cancer (MCRC): results of a randomized phase III trial by the Gruppo Oncologico Nord Ovest (G.O.N.O.). Proc Am Soc Clin Oncol. 2006;24:149a.
Hejna M, Kornek GV, Raderer M, et al. Reinduction therapy with the same cytostatic regimen in patients with advanced colorectal cancer. Br J Cancer. 1998;78:760–4.
Maughan TS, James RD, Kerr DJ, et al. Comparison of intermittent and continuous palliative chemotherapy for advanced colorectal cancer: a multicentre randomised trial. Lancet. 2003;361:457–64.
Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer—a GERCOR study. J Clin Oncol. 2006;24:394–400.
Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol. 2009;27:5727–33.
Perez-Staub N, Chibaudel B, Figer A, et al. Who can benefit from chemotherapy holidays after first-line therapy for advanced colorectal cancer? A GERCOR study. J Clin Oncol. 2008;26(Suppl):4037.
Adams RA, Meade AM, Seymour MT, et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol. 2011;12:642–53.
Labianca R, Floriani I, Cortesi E, et al. Alternating versus continuous “FOLFIRI” in advanced colorectal cancer (ACC): a randomized “GIS-CAD” trial. Proc Am Soc Clin Oncol. 2006;24 Suppl 18:147a.
Yeoh C, Chau I, Cunningham D, et al. Impact of 5-fluorouracil rechallenge on subsequent response and survival in advanced colorectal cancer: pooled analysis from three consecutive randomized controlled trials. Clin Colorectal Cancer. 2003;3:102.
de Gramont A, Buyse M, Abrahantes JC, et al. Reintroduction of oxaliplatin is associated with improved survival in advanced colorectal cancer. J Clin Oncol. 2007;25:3224.
Townsend AR, Bishnoi S, Broadbridge V, et al. Rechallenge with oxaliplatin and fluoropyrimidine for metastatic colorectal carcinoma after prior therapy. J Clin Oncol. 2013;36:49.
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42.
Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007;25:4779–86.
Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol. 2008;26(4):689.
Stathopoulos GP, Batziou C, Trafalis D, et al. Treatment of colorectal cancer with and without bevacizumab: a phase III study. Oncology. 2010;78(5–6):376.
Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–9.
Macedo LT, da Costa Lima AB, Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer. 2012;12:89. Important metanalysis documenting the role of bevacizumab in first line treatment with different chemoregimens.
Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–705.
Vincenzi B, Santini D, Russo A, et al. Bevacizumab in association with de Gramont 5-fluorouracil/folinic acid in patients with oxaliplatin-, irinotecan-, and cetuximab-refractory colorectal cancer: a single-center phase 2 trial. Cancer. 2009;115(20):4849.
Heinemann V, Fischer von Weikersthal L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10). Well documented trial on the advantage of anti-EGFR vs anti-VEGF combinations rates in treatment naïve KRAS WT patients in terms of OS.
Lenz H, Niedzwiecki D, Innocenti F, et al. CALGB/SWOG 80405: PHASE III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with expanded ras analyses untreated metastatic adenocarcinoma of the colon or rectum (MCRC). ESMO 2014 Abstract 501O. Updated results on extended RAS WT patient population proving equivalence of anti-VEGF and anti-EGFR combinations in untreated mCRC patients in phase III setting; these conflicting results with those from the FIRE III study are subject of extensive discussions and translational research.
Parsons BL, Myers MB. Personalized cancer treatment and the myth of KRAS wild-type colon tumors. Discov Med. 2013;15(83):259–67. Excellent review discussing th the role of Kras in mCRC and indicating that represents only the first step in the translational labyrinth.
Hegewisch S, Becker DE. Maintenance strategy with fluoropyrimidines (FP) plus bevacizumab (Bev), Bev alone or no treatment, following a 24-week first-line induction with FP, oxaliplatin (Ox) and Bev for pts with metastatic colorectal cancer: mature data and subgroup analysis of the AIO KRK 0207 phase III study. ESMO 2014; abstr 498O. This study documents in a phase III design the importance of maintenance anti VEGF containing treatment compared to observation for progression free interval but not for survival; markers like RAS/BRAF may be useful for further selection of patients.
Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–9.
Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31.
Miles D, Harbeck N, Escudier B, et al. Disease course patterns after discontinuation of bevacizumab: pooled analysis of randomized phase III trials. J Clin Oncol. 2011;29:83–8. This study clearly demonstrates the lack of rebound effect of bevacizumab intermittent use.
Saltz LB, Lenz HJ, Kindler HL, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol. 2007;25(29):4557.
Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672.
Chibaudel B, Tournigand C, Samson B, et al. Bevacizumab-erlotinib as maintenance therapy in metastatic colorectal cancer. Final results of the GERCOR DREAM study. ESMO 2014; abstract 497O. This study positively validates for the first time the combination of an AntiVEGF MAB with an antiEGFR TKI independently of KRAS status.
Giantonio BJ, Catalano PJ, Meropol NJ, et al. High-dose bevacizumab improves survival when combined with FOLFOX4 in previously treated advanced colorectal cancer: results from the Eastern Cooperative Oncology Group (ECOG) study E3200. Proc Am Soc Clin Oncol. 2005;23 suppl 16:1a.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Colon Cancer V.3. 2013.
Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26(33):5326.
Cartwright TH, Yim YM, Yu E, et al. Survival outcomes of bevacizumab beyond progression in metastatic colorectal cancer patients treated in US community oncology. Clin Colorectal Cancer. 2012;11(4):238.
Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. First randomized phase III trial for bevacizumab continuation after progression on regimen containing bevacizumab and changing the backbone chemotherapy.
Kubicka S, Greil R, André T, et al. Bevacizumab plus chemotherapy continued beyond first progression in patients with metastatic colorectal cancer previously treated with bevacizumab plus chemotherapy: ML18147 study KRAS subgroup findings. Ann Oncol. 2013;24(9):2342–9.
Petrelli F, Barni S. Anti-EGFR agents for liver metastases. Resectability and outcome with anti-EGFR agents in patients with KRAS wild-type colorectal liver-limited metastases: a meta-analysis. Int J Colorectal Dis. 2012;27(8):997–1004.
Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31(16):1931–8.
Folprecht G, Gruenberger T, Bechstein W, Raab HR, Weitz J, Lordick F, et al. Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann Oncol. 2014;25(5):1018–25.
B Nordlinger, GJ. Poston and RM. Goldberg. Should the results of the new EPOC trial change practice in the management of patients with resectable metastatic colorectal cancer confined to the liver? JCO 2014(58):3989
Giordano G, Febbraro A, Venditti M, et al. Targeting angiogenesis and tumor microenvironment in metastatic colorectal cancer: role of aflibercept. Gastroenterology Research and Practice. Vol. 2014
Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. JCO. 2012;30:3499–506. First randomized phase III trial positively validating the role of aflibercept after progression on oxaliplatin regimen, setting a new standard of care.
Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12.
Modest DP, Stintzing S, Fischer Von Weikersthal L, et al. Second-line therapies in patients with KRAS wild-type metastatic colorectal cancer (mCRC) after first-line therapy with FOLFIRI in combination with cetuximab or bevacizumab in the AIO KRK0306 (FIRE 3) trial. J Clin Oncol. 2014;32:5 s, suppl; abstr 3558
Compliance with Ethics Guidelines
Conflict of Interest
Konstantinos Papadimitriou, Christian Rolfo, Elien DeWaelle, Mick Van De Wiel, Jan Van Den Brande, Sevilay Altintas, Manon Huizing, Pol Specenier, and Marc Peeters declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Lower Gastrointestinal Cancers
Rights and permissions
About this article
Cite this article
Papadimitriou, K., Rolfo, C., Dewaele, E. et al. Incorporating Anti-VEGF Pathway Therapy as a Continuum of Care in Metastatic Colorectal Cancer. Curr. Treat. Options in Oncol. 16, 18 (2015). https://doi.org/10.1007/s11864-015-0333-9
Published:
DOI: https://doi.org/10.1007/s11864-015-0333-9