Opinion statement
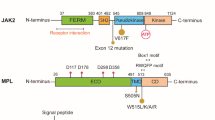
Originally described by Dameshek in 1951, myeloproliferative disorders are today classified as myeloproliferative Neoplasms (MPNs) in WHO’s Classification of Tumors of Hematopoietic and Lymphoid Tissues. The term includes a range of conditions, [ie, BCR-ABL-positive chronic myelogenous leukemia (CML), chronic neutrophilic leukemia (CNL), polycythemia vera (PV), primary myelofibrosis (PMF), essential thromobocythemia (ET), chronic eosinophilic leukemia not otherwise specified (CEL-NOS), mastocytosis, and unclassifiable myeloproliferative neoplasm]. In the specific case of CML, a better understanding of the pathogenesis and pathophysiology of the disease has led to a targeted therapy. The presence of chromosome Philadelphia, t(9;22)(q34;11) results in the oncogene BCR-ABL, which characterizes the disease; this molecular rearrangement gives rise to a tyrosine-kinase, which in turn triggers the proliferation of the myeloid line through the activation of the signaling pathways downstream. Tyrosine-kinase inhibitors (TKIs) have altered the therapy and monitoring of CML patients and improved both their prognosis and quality of life. In 2005, various groups of investigators described a new point mutation of the gene JAK2 associated to MPNs. Although the presence of this mutation has led to a modification in the diagnostic criteria of these conditions, the impact of the use of JAK2 inhibitors on the prognosis and course of the disease continues to be controversial.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372–5.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues. 4th ed. Lyon: WHO Press; 2008.
Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics. Cancer J Clin. 2004;54:8–29.
Johansson P. Epidemiology of the myeloproliferative disorders polycythemia vera and essential thrombocytemia. Semin Thromb Hemost. 2006;32:171–3.
Johansson P, Kutti J, Andreasson B, Safai-Kutti S, Vilen L, Wedel H, et al. Trends in the incidence of chronic Philadelphia chromosome negative (Ph-) myeloproliferative disorders in the city of Goteborg, Sweden, during 1983-99. J Intern Med. 2004;256:161–5.
Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109:68.
Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118:401. This study validates the QOL assessment form in MPN.
Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30:4098.
Tefferi A, Cervantes F, Mesa R, Passamonti F, Verstovsek S, Vannucchi AM, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122:1395–8. This study revises response criteria in MPN.
Nowell PC, Hungerford DA. A minute chromosome in human chronic granulocytic leukemia. Science. 1960;142:1497.
Rowley JD. Letter. A new consistent abnormality in chronic myelogenous leukemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–3.
Bartram C, Klein A, Hagemeijer A, Agthoven T, Geurts van Kessel A, Bootsma D, et al. Translocation of c-abl oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukemia. Nature. 1983;306:277–80.
Groffen J, Stephenson JR, Heisterkamp N, Klein A, Bartram C, Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, BCR, on chromosome 22. Cell. 1984;36:93–9.
Quintas Cardama A, Kantarjian H, Cortes J. Molecular biology and cytogenetics of chronic myeloid leukemia. Neoplastic Diseases of the Blood. 5th Ed. Springer. 2012;91–102.
Daley GQ, Van Etten RA, Baltimora D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–30.
Druker B, Tamura S, Buchdunger E, Ohno S, Bagby G, Lydon N. Preclinical evaluation of a selective inhibitor of the ABL tyrosine kinase as a therapeutic agent for chronic myelogenous leukemia. ASH Annual Meeting Abstracts. Blood. 1995;601a. [Abstract 2392].
Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. For the International STI571 CML study group. N Engl J Med. 2002;346:645–54.
O′Brien S, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low–dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004.
Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–32.
Vainshenker W, Constantinescu SN. A unique activation mutation in JAK2 (V617F) is at the origin of polycythemia vera and allows a new classification of myeloproliferative diseases. Hematology Am Soc Hematol Educ Program. Education Book January 1, vol 2005;2005;195–200.
James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signaling causes polycythemia vera. Nature. 2005;434:1144–8.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90.
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97.
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61.
Neubauer H, Cumano A, Müller M, Wu H, Huffstadt U, Pfeffer K. JAK2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409.
Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. JAK2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–95.
Staerk J, Constantinescu S. The JAK-STAT pathway and hematopoietic stem cells from the JAK2 V617F perspective. JAK-STAT. 2012;1:184–90.
Garcon L, Rivat C, James C, Lacout C, Camara-Clayette V, Ugo V, et al. Constitutive activation of STAT5 and BCL-xL overexpression can induce endogenous erythroid colony formation in human primary cells. Blood. 2006;108:1551–4.
Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–8.
Vizmanos JL, Ormazabal C, Larrayoz MJ, Cross NC, Calasanz MJ. JAK2 V617F mutation in classic chronic myeloproliferative diseases: a report on a series of 349 patients. Leukemia. 2006;20:534–5.
Ceesay M, Lea N, Ingram W, Westwood N, Gaken J, Mohamedali A, et al. The JAK2 V617Fmutation is rare in RARS but common in RARS-T. Leukemia. 2006;20:2060–1.
Levine R, Loriaux M, Huntly B, Loh M, Beran M, Stoffregen E, et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood. 2005;106:3377–9.
Stijnis C, Kroes W, Balkassmi S, Marijt E, van Rossum A, Bakker E, et al. No evidence for JAK2(V617F) mutation in monoclonal B cells in 2 patients with polycythemia vera and concurrent monoclonal B cell disorder. Acta Haematol. 2012;128:183–6.
Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–68.
Wang JY, Ai XF, Xu JQ, Li QH, Xu ZF, Qin TJ, et al. JAK2 exon 12 mutations in patients with Philadelphia (Ph) chromosome-negative myeloproliferative neoplasms. Zhonghua Xue Ye Xue Za Zhi. 2012;33:705–9.
Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472–6.
Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270.
Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J, et al. Ratio of mutant JAK2-V617Fto wild-type JAK2 determines the MPD phenotypesin transgenic mice. Blood. 2008;111:3931–40.
Vannucchi A, Antonioli E, Guglielmelli P, Pardanani A, Tefferi A. Clinical correlates of JAK2V617F presence or allele burden in myeloproliferative neoplasms: a critical reappraisal. Leukemia. 2008;22:1299–307.
Austin SK, Lambert JR. The JAK2V617F mutation and thrombosis. Br J Haematol. 2008;143:307–20.
Alvarez-Larrán A, Bellosillo B, Martínez-Avilés L, Saumell S, Salar A, Abella E, et al. Postpolycythaemic myelofibrosis: frequency and risk factors for this complication in 116 patients. Br J Haematol. 2009;146:504–9.
Vannucchi AM, Antonioli E, Guglielmelli P, Rambaldi A, Barosi G, Marchioli R, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110:840–6.
Finazzi G, Caruso V, Marchioli R, Capnist G, Chisesi T, Finelli C, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood. 2005;105:2664–70.
Kreft A, Büche G, Ghalibafian M, Buhr T, Fischer T, Kirkpatrick CJ. The incidence of myelofibrosis in essential thrombocythaemia, polycythemia vera and chronic idiopathic myelofibrosis: a retrospective evaluation of sequential bone marrow biopsies. Acta Haematol. 2005;113:137–43.
Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342:1255–65.
Cross N. Genetic and epigenetic complexity in myeloproliferative neoplasms. Hematol Am Soc Hematol Educ Prog. 2011;2011:208–14.
Klidajian JL. New approaches to therapy for myeloproliferative neoplasms. Hematol Educ. 2012;6:279–84.
Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis N Engl J Med. 2010;363:1117–27. Analysis of safety and efficacy of ruxolitinib in MF.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. Phase III study for ruxolitinib in MF.
Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib vs best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98. Phase III study for ruxolitinib in MF.
Mesa RA, Gotlib J, Gupta V, Catalano JV, Deininger MW, Shields AL, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2013;31:1285–92. This study reports impact of ruxolitinib in QOL.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, Dipersio JF, et al. Efficacy, safety and survival with ruxolitinib treatment in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Blood. 2013;122(25):4047–4053.
Cervantes F, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Sirulnik A, Stalbovskaya V, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a Phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Haematol. 1 2013;98(12):1865–1871.
Harrison CN, Mesa RA, Kiladjian JJ, Al-Ali HK, Gisslinger H, Knoops L, et al. Health-related quality of life and symptoms in patients with myelofibrosis treated with ruxolitinib vs best available therapy. Br J Haematol. 2013;162:229–39.
Kvasnicka HM, Thiele J, Bueso-Ramos CE, Hou K, Cortes JE, Kantarjian HM, et al. Exploratory analysis of the effect of ruxolitinib on bone marrow morphology in patients with myelofibrosis. ASCO Annual Meeting Abstracts. J Clin Oncol. 2013;31(Suppl):7030.
Vannucchi A, Kiladjian JJ, Gisslinger H, Passamonti F, Al-Ali HK, Sirulnik LA, et al. Reductions in JAK2V617F allele burden with ruxolitinib treatment in COMFORT-II, a Phase III study comparing the safety and efficacy of ruxolitinib to best available therapy (BAT). J Clin Oncol. 2012;30(Suppl). [Abstract 6514].
Verstovsek S, Passamonti P, Rambaldi A, Giovanni Barosi G, Peter J, Rosen PJ, et al. Long-term efficacy and safety results from a Phase II study of ruxolitinib in patients with polycythemia vera. ASH Annual Meeting Abstracts. Blood. 2012;120:804.
Talpaz M, Jamieson C, Gabrail NY, Lebedinsky C, Gao G, Liu F, et al. A Phase II randomized dose-ranging study of the JAK2-selective inhibitor sar302503 in patients with intermediate-2 or high-risk primary myelofibrosis (MF), post-polycythemia vera (PV), MF, or post-essential thrombocythemia (ET) MF. ASH Annual Meeting Abstracts. Blood. 2012;120. [Abstract 2837].
Pardanani A, Gotlib J, Jamieson C, Cortes JE, Talpaz M., Stone R, et al. SAR302503: interim safety, efficacy and long-term impact on JAK2 V617F allele burden in a Phase I/II study in patients with myelofibrosis. ASH Annual Meeting Abstracts. Blood. 2011;118. [Abstract 3838].
Deeg HJ, Odenike O, Scott BL, Estrov Z, Cortes JE, Thomas DA, et al. Phase II study of SB1518, an orally available novel JAK2 inhibitor, in patients with myelofibrosis. J Clin Oncol. 2011;29(Suppl). [Abstract 6515].
Pardanani A, Gotlib J, Gupta V, Roberts AW, Wadleigh M, Sirhan S, et al. An expanded multicenter Phase I/II study of CYT387, a JAK- 1/2 inhibitor for the treatment of myelofibrosis. ASH Annual Meeting Abstracts. Blood. 2011;118. [Abstract 3849].
Pardanani AD, Caramazza D, George G, Lasho TL, Hogan WJ, Litzow MR, et al. Safety and efficacy of CYT387, a JAK-1/2 inhibitor, for the treatment of myelofibrosis. J Clin Oncol. 29:2011(Suppl). [Abstract 6514].
Verstovsek S, Mesa RA, Kloeker-Rhoades S, Giles JL, Pitou C, Jones E, et al. Phase I study of the JAK2 V617F Inhibitor, LY2784544, in patients with myelofibrosis (MF), polycythemia Vera (PV), and essential thrombocythemia (ET). ASH Annual Meeting Abstracts. Blood. 2011;118. [Abstract 2814].
Compliance with Ethics Guidelines
Conflict of Interest
Pablo J. Muxí had travel/accommodations expenses covered or reimbursed by Novartis. Ana Carolina Oliver declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muxí, P.J., Oliver, A.C. Jak-2 Positive Myeloproliferative Neoplasms. Curr. Treat. Options in Oncol. 15, 147–156 (2014). https://doi.org/10.1007/s11864-014-0279-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-014-0279-3