Abstract
Objective
To observe the preventive and therapeutic effects of diosgenin on retinoic acid-induced osteoporosis in rats.
Methods
A total 50 Sprague-Dawley rats were randomly divided into 5 groups: control group, model group (osteoporosis rats), low (10 mg kg−1), middle (30 mg kg−1), and high-dose diosgenin (90 mg kg−1)-treated groups. The osteoporosis rats model was induced by retinoic acid. The BMD and physical parameters of femoral including length, wet weight, and dry weight in each group were measured. The hematoxylin–eosin staining was used for bone histomorphology analysis. Besides, the bone calcium (Ca) and phosphorus (P) contents were measured. In order to detect the biochemical index in different treatment groups, the serum tartrate-resistant acid phosphatase (TRAP), alkaline phosphatase (ALP), estradiol, and osteocalcin were compared among different groups.
Results
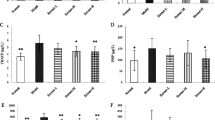
The osteoporosis rat model was successfully induced by retinoic acid. Compared with the model group, the lessening of femoral length and weight and the loss of BMD were significantly improved in diosgenin groups. Both contents of Ca and P were much more increased when induced by retinoic acid (p < 0.05). The estradiol and osteocalcin levels in the middle and high-dose treatment groups were significantly higher than that of the model group, while the ALP and TRAP levels were much lower than the model group (p < 0.05).
Conclusion
Diosgenin can prevent the loss of bone in experimental rats. The mechanism may be that it improves the level of estrogenic hormone of estradiol and inhibits the high bone turnover.





Similar content being viewed by others
References
Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9(8):1137–1141
Kanis J, Black D, Cooper C, Dargent P, Dawson-Hughes B, De Laet C, Delmas P, Eisman J, Johnell O, Jonsson B (2002) A new approach to the development of assessment guidelines for osteoporosis. Osteoporos Int 13(7):527–536
Hodgson S, Watts N, Bilezikian J, Clarke B, Gray T, Harris D, Johnston C Jr, Kleerekoper M, Lindsay R, Luckey M (2003) American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr Pract 9(6):544
Ammann P, Rizzoli R (2003) Bone strength and its determinants. Osteoporos Int 14(3):13–18
Wood AJ, Riggs BL, Melton LJ III (1992) The prevention and treatment of osteoporosis. N Engl J Med 327(9):620–627
Ueda H, Yamazaki C, Yamazaki M (2002) Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol Pharm Bull 25(9):1197–1202
Gryglewski RJ, Korbut R, Robak J, Świȩs J (1987) On the mechanism of antithrombotic action of flavonoids. Biochem Pharmacol 36(3):317–322
Chiba H, Uehara M, Wu J, Wang X, Masuyama R, Suzuki K, Kanazawa K, Ishimi Y (2003) Hesperidin, a citrus flavonoid, inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice. J Nutr 133(6):1892–1897
Kim T-H, Jung JW, Ha BG, Hong JM, Park EK, Kim H-J, Kim S-Y (2011) The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J Nutr Biochem 22(1):8–15
Raju J, Patlolla JM, Swamy MV, Rao CV (2004) Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomark Prev 13(8):1392–1398
Gupta A, Gupta R, Lal B (2001) Effect of Trigonella foenum-graecum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: a double blind placebo controlled study. J Assoc Phys India 49:1057–1061
Corbiere C, Liagre B, Bianchi A, Bordji K, Dauça M, Netter P, Beneytout J-L (2003) Different contribution of apoptosis to the antiproliferative effects of diosgenin and other plant steroids, hecogenin and tigogenin, on human 1547 osteosarcoma cells. Int J Oncol 22(4):899–906
Liu M-J, Wang Z, Ju Y, Wong RN-S, Wu Q-Y (2005) Diosgenin induces cell cycle arrest and apoptosis in human leukemia K562 cells with the disruption of Ca2+ homeostasis. Cancer Chemother Pharmacol 55(1):79–90
Higdon K, Scott A, Tucci M, Benghuzzi H, Tsao A, Puckett A, Cason Z, Hughes J (2001) The use of estrogen, DHEA, and diosgenin in a sustained delivery setting as a novel treatment approach for osteoporosis in the ovariectomized adult rat model. Biomed Sci Instrum 37:281
Yen ML, Su JL, Chien CL, Tseng KW, Yang CY, Chen WF, Chang CC, Kuo ML (2005) Diosgenin induces hypoxia-inducible factor-1 activation and angiogenesis through estrogen receptor-related phosphatidylinositol 3-kinase/Akt and p38 mitogen-activated protein kinase pathways in osteoblasts. Mol Pharmacol 68(4):1061–1073
Wu BXB, Huang T, Wang J (1996) A model of osteoporosis induced by retinoic acid in male Wistar rats. Acta Pharm Sin 31(4):241
Wei MYZ, Li P, Zhang Y, Sse WC (2007) Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am J Chin Med 35(04):663–667
Liao EY, Luo XH, Wang WB, Wu XP, Zhou HD, Dai RC, Liao HJ, Yang C (2003) Effects of different nylestriol/levonorgestrel dosages on bone metabolism in female Sprague-Dawley rats with retinoic acid-induced osteoporosis. Endocr Res 29(1):23–42
Xu P, Guo X, Zhang YG, Li YF, Cao JL, Xiong YM (2005) The effect of retinoic acid on induction of osteoporotic model rats and the possible mechanism. Sichuan Da Xue Xue Bao Yi Xue Ban 36:229–232
Fahmy SR, Soliman AM (2009) Oxidative stress as a risk factor of osteoporotic model induced by vitamin A in rats. Aust J Basic Appl Sci 3:1559–1568
Hough SAL, Muir H, Gelderblom D, Jenkins G, Kurasi H, Slatopolsky E, Bergfeld MA, Teitelbaum SL (1988) Effects of hypervitaminosis A on the bone and mineral metabolism of the rat. Endocrinology 122:2933–2939
Oršolić N, Goluža E, Đikić D, Lisičić D, Sašilo K, Rođak E, Jeleč Ž, Lazarus M, Orct T (2013) Role of flavonoids on oxidative stress and mineral contents in the retinoic acid-induced bone loss model of rat. Eur J Nutr 53:1217–1227. doi:10.1007/s00394-013-0622-7
Zhang Z, Song C, Fu X, Liu M, Li Y, Pan J, Liu H, Wang S, Xiang L, Xiao GG, Ju D (2014) High-dose diosgenin reduces bone loss in ovariectomized rats via attenuation of the RANKL/OPG ratio. Int J Mol Sci 15(9):17130–17147
Price P, Parthemore J, Deftos L (1980) New biochemical marker for bone metabolism. Measurement by radioimmunoassay of bone GLA protein in the plasma of normal subjects and patients with bone disease. J Clin Investig 66(5):878
Zhang Y, Yu L, Ao M, Jin W (2006) Effect of ethanol extract of Lepidium meyenii Walp. on osteoporosis in ovariectomized rat. J Ethnopharmacol 105(1):274–279
Zhang QYQL, Huang BK, Wang Y, Wang H, Chen L (2003) Effects and mechanisms of osthole on sciatica induced by lumber disc herniation. Chin Pharm J 5:101–104
LE Bu SY, Frankin M, Marlow D, Brachett DJ, Boldrin EA (2007) Comparison of dried plum supplementation and intermittent PTH in restoring bone in osteopenic orchidectomized rats. Osteoporos Int 18:931–942
Farley JR, Stilt-Coffing B (2001) Apoptosis may determine the release of skeletal alkaline phosphatase activity from human osteoblast-line cells. Calcif Tissue Int 68(1):43–52. doi:10.1007/s002230001181
Minkin C (1982) Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int 34(1):285–290
Deyhim F, Garica K, Lopez E, Gonzalez J, Ino S, Garcia M, Patil BS (2006) Citrus juice modulates bone strength in male senescent rat model of osteoporosis. Nutrition 22(5):559–563
Bharadwaj SNA, Betageri GV, Prasadarao NV, Naidu AS (2009) Milk ribonuclease-enriched lactoferrin induces positive effects on bone turnover markers in postmenopausal women. Osteoporos Int 20:1603–1611
Born AK, Lischer S, Maniura-Weber K (2012) Watching osteogenesis: life monitoring of osteogenic differentiation using an osteocalcin reporter. J Cell Biochem 113(1):313–321
Filip R, Possemiers S, Heyerick A, Pinheiro I, Raszewski G, Davicco MJ, Coxam V (2014) Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J Nutr Health Aging 19:77–86. doi:10.1007/s12603-014-0480-x
Fernández-Real JM, Bulló M, Moreno-Navarrete JM, Ricart W, Ros E, Estruch R, Salas-Salvado J (2012) A Mediterranean diet enriched with olive oil is associated with higher serum total osteocalcin levels in elderly men at high cardiovascular risk. J Clin Endocrinol Metab 97(10):3792–3798
Stepan JJPJ, Presl J (1987) Bone loss and biochemical indices of bone remodeling in surgically induced post-menopausal women. Bone 8:279–284
Ferretti M, Bertoni L, Cavani F, Benincasa M, Sena P, Carnevale G, Zavatti M, Di Viesti V, Zanoli P, Palumbo C (2012) Structural and histomorphometric evaluations of ferutinin effects on the uterus of ovariectomized rats during osteoporosis treatment. Life Sci 90(3):161–168
Rao LG, Krishnadev N, Banasikowska K, Rao AV (2003) Lycopene I—effect on osteoclasts: lycopene inhibits basal and parathyroid hormone-stimulated osteoclast formation and mineral resorption mediated by reactive oxygen species in rat bone marrow cultures. J Med Food 6(2):69–78
Mandadi K, Ramirez M, Jayaprakasha GK, Faraji B, Lihono M, Deyhim F, Patil BS (2009) Citrus bioactive compounds improve bone quality and plasma antioxidant activity in orchidectomized rats. Phytomedicine 16(6):513–520
Kono R, Okuno Y, K-i Inada, Tokuda A, Hashizume H, Yoshida M, Nakamura M, Utsunomiya H (2011) A Prunus mume extract stimulated the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biosci Biotechnol Biochem 75(10):1907–1911
Garrett I, Boyce B, Oreffo R, Bonewald L, Poser J, Mundy G (1990) Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Investig 85(3):632
Shishodia S, Aggarwal B (2005) Diosgenin inhibits osteoclastogenesis, invasion, and proliferation through the downregulation of Akt, IκB kinase activation and NF-κB-regulated gene expression. Oncogene 25(10):1463–1473
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, S., Niu, F., Xu, CY. et al. Diosgenin prevents bone loss on retinoic acid-induced osteoporosis in rats. Ir J Med Sci 185, 581–587 (2016). https://doi.org/10.1007/s11845-015-1309-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-015-1309-2