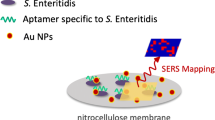
Abstract
The detection of Salmonella Poona from cantaloupe cubes and E. coli O157:H7 from lettuce has been explored by using a filtration method and surface-enhanced Raman spectroscopy (SERS) based on vancomycin-functionalized silver nanorod array substrates. It is found that with a two-step filtration process, the limit of detection (LOD) of Salmonella Poona from cantaloupe cubes can be as low as 100 CFU/mL in less than 4 h, whereas the chlorophyll in the lettuce causes severe SERS spectral interference. To improve the LOD of lettuce, a three-step filtration method with a hydrophobic filter is proposed. The hydrophobic filter can effectively eliminate the interferences from chlorophyll and achieve a LOD of 1000 CFU/mL detection of E. coli O157:H7 from lettuce samples within 5 h. With the low LODs and rapid detection time, the SERS biosensing platform has demonstrated its potential as a rapid, simple, and inexpensive means for pathogenic bacteria detection from fresh produce.






Similar content being viewed by others
References
L.R. Beuchat, J. Food Prot. 59, 204 (1996).
K.R. Matthews, Microbiology of Fresh Produce (Washington, DC: American Society for Microbiology, 2006), pp. 1–19.
C.S. DeWaal and F. Bhuiya, Outbreaks by the Numbers: Fruits and Vegetables 1990–2005 (Washington, DC: Center for Science and the Public Interest, 2007).
M.F. Lynch, R.V. Tauxe, and C.W. Hedberg, Epidemiol. Infect. 137, 307 (2009).
U. Buchholz, H. Bernard, D. Werber, M.M. Böhmer, C. Remschmidt, H. Wilking, Y. Deleré, M. an der Heiden, C. Adlhoch, J. Dreesman, J. Ehlers, S. Ethelberg, M. Faber, C. Frank, G. Fricke, M. Greiner, M. Höhle, S. Ivarsson, U. Jark, M. Kirchner, J. Koch, G. Krause, P. Luber, B. Rosner, K. Stark, and M. Kühne, New Engl. J. Med. 365, 1763 (2011).
C.N. Berger, S.V. Sodha, R.K. Shaw, P.M. Griffin, D. Pink, and G. Frankel, Environ. Microbiol. 12, 2385 (2010).
M.P. Doyle and M.C. Erickson, J. Appl. Microbiol. 105, 317 (2008).
L.J. Harris, J.N. Farber, L.R. Beuchat, M.E. Parish, T.V. Suslow, E.H. Garrett, and F.F. Busta, Compr. Rev. Food Sci. Food Saf. 2, 78 (2003).
CDC, Multistate Outbreak of Salmonella Typhimurium and Salmonella Newport Infections Linked to Cantaloupe (Final Update) (Atlanta: Centers for Disease Control and Prevention, 2012).
CDC, Reports of Selected E. coli Outbreak Investigations (Atlanta: Centers for Disease Control and Prevention, 2014).
J.T. Fox, J.S. Drouillard, X. Shi, and T.G. Nagaraja, J. Anim. Sci. 87, 1304 (2009).
S. Bouguelia, Y. Roupioz, S. Slimani, L. Mondani, M.G. Casabona, C. Durmort, T. Vernet, R. Calemczuk, and T. Livache, Lab Chip 13, 4024 (2013).
R.D. Hoelzle, B. Virdis, and D.J. Batstone, Biotechnol. Bioeng. 111, 2139 (2014).
A. Huang, Z. Qiu, M. Jin, Z. Shen, Z. Chen, X. Wang, and J.W. Li, Int. J. Food Microbiol. 185, 27 (2014).
S. Furukawa, Y. Akiyoshi, G.A. O’Toole, H. Ogihara, and Y. Morinaga, Int. J. Food Microbiol. 138, 176 (2010).
H.S. Eom, B.H. Hwang, D.H. Kim, I.B. Lee, Y.H. Kim, and H.J. Cha, Biosens. Bioelectron. 22, 845 (2007).
S.E. Frima and L. Zeiri, J. Raman Spectrosc. 40, 277 (2009).
K. Kneipp, Y. Wang, H. Kneipp, L.T. Perelman, I. Itzkan, R.R. Dasari, and M.S. Feld, Phys. Rev. Lett. 78, 1667 (1997).
S.M. Nie and S.R. Emery, Science 275, 1102 (1997).
S.B. Chaney, S. Shanmukh, R.A. Dluhy, and Y.P. Zhao, Appl. Phys. Lett. 87, 031908 (2005).
J.D. Driskell, S. Shanmukh, Y. Liu, S.B. Chaney, X.J. Tang, Y.P. Zhao, and R.A. Dluhy, J. Phys. Chem. C 112, 895 (2008).
S. Shanmukh, L. Jones, J. Driskell, Y.P. Zhao, R. Dluhy, and R.A. Tripp, Nano Lett. 6, 2630 (2006).
Y.P. Zhao, S.B. Chaney, S. Shanmukh, and R.A. Dluhy, J. Phys. Chem. B 110, 3153 (2006).
J.D. Driskell, Y. Zhu, C.D. Kirkwood, Y.P. Zhao, R.A. Dluhy, and R.A. Tripp, PLoS One 5, e10222 (2010).
S. Shanmukh, L. Jones, Y.P. Zhao, J. Driskell, R. Tripp, and R. Dluhy, Anal. Bioanal. Chem. 390, 1551 (2008).
H. Chu, Y. Huang, and Y. Zhao, Appl. Spectrosc. 62, 922 (2008).
X. Wu, S. Gao, J.S. Wang, H. Wang, Y.W. Huang, and Y. Zhao, Analyst 137, 4226 (2012).
X. Wu, J. Chen, B. Park, Y. Huang, and Y. Zhao, Advances in Applied Nanotechnology for Agriculture (Washington, DC: American Chemical Society, 2013), pp. 85–108.
X. Du, H. Chu, Y. Huang, and Y. Zhao, Appl. Spectrosc. 64, 781 (2010).
J.D. Driskell, A.G. Seto, L.P. Jones, S. Jokela, R.A. Dluhy, Y.P. Zhao, and R.A. Tripp, Biosens. Bioelectron. 24, 917 (2008).
X. Wu, C. Xu, R.A. Tripp, Y. Huang, and Y. Zhao, Analyst 138, 3005 (2013).
Y.J. Liu, H.Y. Chu, and Y.P. Zhao, J. Phys. Chem. C 114, 8176 (2010).
X.M. Wu, Y.W. Huang, B. Park, R.A. Tripp, and Y.P. Zhao, Talanta 139, 96 (2015).
M. Manjare and Y. Ting, Wu, B. Yang, and Y.P. Zhao. Appl. Phys. Lett. 104, 054102 (2014).
J.G. Fan and Y.P. Zhao, Langmuire 26, 8245 (2010).
D.I. Arnon, Plant Physiol. 24, 1 (1949).
Acknowledgements
This research was partially founded by the National Science Foundation under contract number CBET-1064228. X.W. and J.C. would like to thank UGA College of Agricultural and Environmental Sciences Experimental Station for their generous financial support. C.H. would like to thank National Natural Science Foundation of China (Grant No. 61575087); Natural Science Foundation of Jiangsu Province, China (Grant No. BK20151164); and the Priority Academic Program Development of Jiangsu Higher Education Institutions for their generous financial support of her research conducted at the University of Georgia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, X., Han, C., Chen, J. et al. Rapid Detection of Pathogenic Bacteria from Fresh Produce by Filtration and Surface-Enhanced Raman Spectroscopy. JOM 68, 1156–1162 (2016). https://doi.org/10.1007/s11837-015-1724-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-015-1724-x