Abstract
Spatiotemporal variation in the availability of food resources may be a determining factor for reproductive success and maintenance of bees, but the extent of these variations is poorly understood. For management and conservation of bees, the first step is to know the behavior and the food resources used. Currently, urban areas are considered refuge zones for bees, and understanding the availability of floral resources and the influence on reproductive processes is very important for management of bees. We used the protocols applied in phenological studies with bees and plant species to evaluate both throughout the year in an urbanized area. At the same time, we used palynology protocols to analyze the pollen material collected from brood cells (food and feces) of immature Centris analis. These protocols allowed to evaluate the availability of floral resources in the studied area and the plant species effectively used by C. analis females to feed immature larvae during the reproductive period. The maximum reproductive period of C. analis was not associated with the highest floral resources availability. However, there was a strong selectivity of pollen in flowers of Malpighia emarginata (Malpighiaceae), which represented more than 59% of all the pollen grains provisioned throughout the year. This means that in the case of more specialized bees like C. analis, the availability of the preferred plants is more important than the overall floral resource availability in the area. Thus, to keep C. analis in the city, it is necessary to maintain or introduce Malpighiaceae species in the urban planning. On the other hand, at least 27% of the plant species found in the study area are pollinated by C. analis, emphasizing the importance of preserving this bee.



Similar content being viewed by others
References
Abrahamczyk S, Gottleuber P, Matauschek C, Kessler M (2011) Diversity and community composition of euglossine bee assemblages (Hymenoptera: Apidae) in western Amazonia. Biodivers Conserv 20:2981–3001
Agostini K, Sazima M (2003) Plantas ornamentais e seus recursos para abelhas no Campus da Universidade Estadual de Campinas, Estado de São Paulo, Brasil. Bragantia 62:335–343.
Aguiar CML, Garófalo CA (2004) Nesting biology of Centris (Hemisiella) tarsata Smith (Hymenoptera, Apidae, Centridini). Ver Bras Zool 21:477–486.
Aguiar AJC, Martins CF (2002) Abelhas e vespas solitárias em ninhos-armadilha na Reserva Biológica Guaribas (Mamanguape, Paraíba, Brasil). Ver Bras Zool 19:101–116.
Aguiar CML, Zanella FCV, Martins CF, Carvalho CAL (2003) Plantas visitadas por Centris spp. (Hymenoptera: Apidae) na Caatinga para obtenção de recursos florais. Neotrop Entomol 32(2):247–259
Aguiar CM, Garófalo CA, Almeida GF (2005) Trap-nesting bees (Hymenoptera, Apoidea) in areas of dry semideciduous forest and caatinga, Bahia, Brazil. Ver Bras Zool 22:1030–1038.
Aguiar CML, Garófalo CA, Almeida GF (2006) Biologia de nidificação de Centris (Hemisiella) trigonoides Lepeletier (Hymenoptera, Apidae, Centridini). Ver Bras Zool 23:323–330.
Aguirres LF, Lens L, Matthysen E (2003) Patterns of roost use by bats in a neotropical savanna: implications for conservation. Biol Conserv 111:435–443
Aleixo KP, de Faria LB, Garófalo CA, Imperatriz-Fonseca VL, Silva CI (2013) Pollen collected and foraging activities of Frieseomelitta varia (lepeletier) (hymenoptera: Apidae) in an urban landscape. Sociobiology 60:266–276. doi:10.13102/sociobiology.v60i3.266-276
Aleixo KP, de Faria LB, Groppo M, Castro MMN (2014) Spatiotemporal distribution of floral resources in a Brazilian city: implications for the maintenance of pollinators, especially bees. Urban For Urban Gree 13:689–696. doi:10.1016/j.ufug.2014.08.002.
Alonso JDS, Silva JF, Garófalo CA (2012) The effects of cavity length on nest size, sex ratio and mortality of ratio and mortality of Centris (Heterocentris) analis (Hymenoptera, Apidae, Centridini). Apidologie 43:436–448
Alves TTL, Mascena VM, Silva JN, Freitas BM (2014) Diversidade de insetos e frequência de abelhas visitantes florais de Serjania lethalis na Chapada do Araripe. Revista Verde 9(4):112–116.
Alves-dos-Santos I, Machado IC, Gaglianone MC (2007) História natural das abelhas coletoras de óleo. Oecol Bras 11:544–557. doi:10.4257/oeco.2007.1104.06.
Amaral-Neto LP, Westerkamp C, Melo GAR (2015) From keel to inverted keel flowers: functional morphology of “upside down” papilionoid flowers and the behavior of their bee visitors. Plant Syst Evol 301:2161. doi:10.1007/s00606-015-1221-2
Azevedo RL, Carvalho CAL, Pereira LL, Nascimento AS (2007) Abelhas (Hymenoptera: Apoidea) visitantes das flores do feijão guandu no Recôncavo Baiano, Brasil. Cienc Rural 37(5):1453–1457.
Bartomeus I, Ascher JS, Wagner D, Danforth BN, Colla S, Kornbluth S, Winfree R (2011) Climate-associated phenological advances in bee pollinators and bee-pollinated plants. P Natl Acad Sci USA 108:20645–20649. doi:10.1073/pnas.1115559108.
Bauermann SG, Radaeski JN, Evaldt ACP, Queiroz EP, Mourelle D, Prieto AR, Silva CI (2013) Pólen nas angiospermas: diversidade e evolução. Ulbra, Canoas
Boaventura YMS, Matthes LAF (1987) Aspectos da biologia da reprodução em plantas ornamentais cultivadas no Estado de São Paulo 1—Dichorisandra thyrsiflora Mikan (Commelinaceae). Acta Bot Bras 1(2):189–199.
Borges LA, Sobrinho MS, Lopes AV (2009) Phenology, pollination, and breeding system of the threatened tree Caesalpinia echinata Lam. (Fabaceae), and a review of studies on the reproductive biology in the genus. Flora 204:111–130
Brito VLG, Pinheiro M, Sazima M (2010) Sophora tomentosa e Crotalaria vitelina (Fabaceae): biologia reprodutiva e interações com abelhas na restinga de Ubatuba, São Paulo. Biota Neotrop 10(1):185–192.
Buchmann SL (1987) The ecology of oil flowers and their bees. Ann Rev Ecol Evol Syst 18:343–369
Buchmann SL, Cane JH (1989) Bees assess pollen returns while sonicating Solanum flowers. Oecologia 81:289–294
Buschini MLT, Wolff LL (2006) Nesting biology of Centris (Hemisiella) tarsata Smith in southern Brazil (Hymenoptera, Apidae, Centridini). Braz J Biol 66:1091–1101
Camargo JMF, Mazucato M (1984) Inventário da apifauna e flora apícola da Ribeirão Preto, SP, Brasil. Dusenia 14:55–87.
Camillo E, Garófalo CA, Serrano JC, Muccillo G (1995) Diversidade e abundância sazonal de abelhas e vespas solitárias em ninhos armadilhas (Hymenoptera: Apocrita: Aculeata). Rev Bras Entomol 39:459–470.
Castro MS (2002) Bee fauna of some tropical and exotic fruits: potencial pollinators and their conversation. In Kevan P, Imperatriz-Fonseca VL (eds) Pollinating bees—the conservation link between agriculture and nature. Ministry of Environment, Brasilia, pp 275–288.
Cesario LF, Gaglianone MC (2008) Floral biology and reproductive phenology of Schinus terebinthifolius Raddi (Anacardiaceae) in the restinga of northern Rio de Janeiro State. Acta Bot Bras 22:828–833.
Coville RE, Frankie GW, Vinson SB (1983) Nests of Centris segregata (Hymenoptera: Anthophoridae) with a review of the nesting habitats of the genus. J Kansas Entomol Soc 56:109–122
Crawley MJ (2005) Statistics: an Introduction using R. Wiley, Chichester
Del-Claro K (2004) Multitrophic relationships, conditional mutualisms, and the study of interaction biodiversity in tropical savannas. Neotrop Entomol 33:665–672
Dórea MC, Aguiar CML, Figueroa LER, Lima CL, Santos FAR (2010) Residual pollen in nests of Centris analis (Hymenoptera, Apidae, Centridini) in an area of caatinga vegetation from Brasil. Oecol Aus 14:232–237. doi:10.4257/oeco.2010.1401.13.
Doyle JJ, Lucknow A (2003) The rest of the iceberg. legume diversity and evolution in a phylogenetic context. Plant Physiol 131(3):900–910
Drummont P, Silva FO, Viana BF (2008) Ninhos de Centris (Heterocentris) terminata Smith (Hymenoptera: Apidae, Centridini) em fragmentos de Mata Atlântica secundária, Salvador, BA. Neotrop Entomol 37:239–246
Erdtman G (1960) The acetolized method. a revised description. Bot Not 54:561–564.
Etcheverry AV, Alemán CET (2005) Reproductive biology of Erythrina falcata (Fabaceae: Papilionoideae). Biotropica 37(1):54–63
Faegri K, Pijl vd (1979) The principles of pollination ecology, 3rd ed. Pergamon Press, New York.
Faria LB, Aleixo KP, Garófalo CA, Imperatriz-Fonseca VL, Silva CI (2012) Foraging of scaptotrigona aff. depilis (Hymenoptera, Apidae) in an Urbanized Area†¯: seasonality in resource availability and visited plants. Psyche (Stuttg). doi:10.1155/2012/630628.
Frankie GW, Opler PA, Bawa KS (1976) Foraging behaviour of solitary bees: Implications for outcrossing of a neotropical forest tree species. J Ecol 64(3):1049–1057
Frankie GW, Thorp RW, Newstrom-Lloyd LE, Rizzardi MA, Barthell JF, Griswold TL, Kim JY, Kappagoda S (1998) Monitoring solitary bees in modified wildland habitats: implications for bee ecology and conservation. Environ Entomol 27:1137–1148
Gaglianone MC (2003) Abelhas da tribo Centridini na Estação Ecológica de Jataí (Luiz Antinio, SP): composição de espécies e interações com flores de Malpighiaceae. In Melo GAR, Santos IA (eds) Apoidea Neotropica: Homenagem aos 90 anos de Jesus Santiago Moure, UNESC, Criciúma, pp 279–284.
Garófalo CA, Martin CF, Alves dos Santos I (2004) The Brazilian solitary bee species caught in trap nests. In Freitas BM, Pereira JO (eds) Solitary bees, conservation, rearing and management for pollination. Imprensa Universitária, Fortaleza, pp 77–84.
Gazola AL, Garófalo CA (2003) Parasitic behavior of Leucospis cayennensis Westwood (Hymenoptera, Leucospidae) and rates of parasitism in populations of Centris (Heterocentris) analis (Fabricius) (Hymenoptera, Apidae, Centridini). J Kansas Entomol Soc 76:131–142
Gazola AL, Garófalo CA (2009) Trap-nesting bees (Hymenoptera: Apoidea) in forest fragments of the state of São Paulo, Brazil. Genet Mol Res 8(2):607–622
Gonçalves CBS, Silva CB, Mota JH, Soares TS (2008) Atividade de insetos em flores de Ocimum gratissimum L. e suas interações com fatores ambientais. Caatinga 21(3):128–133.
Heard TA (1999) The role of stingless bees in crop pollination. Annu Rev Entomol 44:183–206
Heithaus ER (1979) Community structure of neotropical flower visiting bees and wasps: diversity and phenology. Ecology 60:190–202
Jha S, Kremen C (2013) Resource diversity and landscape-level homogeneity drive native bee foraging. Proc Natl Acad Sci USA 110(2):555–558. doi:10.1073/pnas.1208682110
Kill LHP, Drumond MA (2001) Biologia floral e sistema reprodutivo de Gliricia sepium (Jacq.) Steud. (Fabaceae—Papilionoidae) na região de Petrolina, Pernambuco. Cienc Rural 31(4):597–601.
Kingha BMT, Fohouo FNT, Ngakou A, Brückner D (2012) Foraging and pollination activities of Xylocopa olivacea (Hymenoptera, Apidae) on Phaseolus vulgaris (Fabaceae) flowers at dang (Ngaoundere-Cameroon). J Agric Ext Rural Dev 4(6):330–339
Kinoshita LS, Torres RB, Forni-Martins ER, Spinelli T, Ahn YJ, Constâncio SS (2006) Composição florística e síndromes de polinização e de dispersão da mata do Sítio São Francisco, Campinas, SP, Brasil. Acta Bot Bras 20:313–327.
Kovach Computing Services (2012) Oriana version 4.0 for windows. Kovach Computing Services, Anglesey
Kremen CN, Williams NM, Aizen MA et al (2007) Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol Lett 10:299–314
Laroca S, Cure JR, Bortoli C (1982) A associação de abelhas silvestres (Hymenoptera, Apoidea) de uma área restrita no interior da cidade de Curitiba (Brasil): uma abordagem biocenótica. Dusenia 17:93–117.
Linsley EG (1958) The ecology of solitary bees. Hilgardia 27:543–591
Lopes TT (2013) Potencial do cipó-uva (Serjania lethalis) como fonte de néctar para a exploração apícola na Chapada do Araripe. Thesis. Universidade Federal do Ceará, Fortaleza.
López-Uribe MM, Oi CA, Del Lama MA (2008) Nectar-foraging behavior of Euglossine bees (Hymenoptera: Apidae) in urban areas. Apidologie 39:410–418
Maurizio A, Louveaux J (1960) Pollens de plantes mellifères d’Europe. Pollens Spores 2:159–182.
Mayer C, Kuhlmann M (2004) Synchrony of pollinators and plants in the winter rainfall area of South Africa—observations from a drought year. T Roy Soc S Afr 59:55–57.
McFrederick Q, LeBuhn G (2006) Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biol Conserv 129:372–382
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176
Mendes FN, Rêgo MMC (2007) Nidificação de centris (Hemisiella) tarsata Smith (Hymenoptera, Apidae, Centridini) em ninhos-armadilha no Nordeste do maranhão, Brasil. Rev Bras Bot 51: 382–388. doi:10.1590/S0085-56262007000300017
Mesquita TMS, Augusto SC (2011) Diversity of trap-nesting bees and their natural enemies in the Brazilian savanna. Trop Zool 24:127–144.
Michener CD (2007) The bees of the word. The Johns Hopkins University Press, Baltimore
Minckley RL, Roulston TH (2006) Incidental mutualisms and pollen specialization among bees. In Waser NM, Ollerton J (eds) Plant-pollinator interactions: from specialization to generalization. The University of Chicago Press, Chicago, pp 69–98
Minckley RL, Cane JH, Kervin L (2000) Origins and ecological consequences of pollen specialization among desert bees. Proc R Soc B Biol Sci 267:265–271.
Montero I, Tormo R (1990) Análisis polínico de mieles de cuatro zonas montañosas de extremadura. Anales de la Asociación de Palinólogos de Lengua Española 5:71–78.
Morato EF, Garcia MVB, Campos LAO (1999) Biologia de centris fabricius (Hymenoptera, Anthophoridae, Centridini) em matas contínuas e fragmentadas na amazônia central. Ver Bras Zool 16:1213–1222.
Morellato LPC, Talora DC, Takahasi A, Bencke CC, Romera EC, Zipparro VB (2000) Phenology of atlantic rain forest trees: a comparative study. Biotropica 32:811–823
Mouga DMDS, Dec E (2012) Catálogo polínico de plantas medicinais apícolas. Dioesc, Florianópolis.
Moure JS (1960) Abelhas da região neotropical descritas por G. Gribodo (Hymenoptera-Apoidea). Bol Uni Paraná 1:1–18.
Navarro L, Medel R (2009) Relationship between floral tube length and nectar robbing in Duranta erecta L. (Verbenaceae). Biol J Linnean Soc 96:392–398.
Nogueira-Couto RH, Pereira JMS, De Jong D (1998) Pollination of Glycine wightii, by Africanized honey bees. J Api Res 37(4):289–291
Nogueira-Neto P (2002) Management of plants to maintain and study pollination bee species, and also to protect vertebrate frugivorous fauna. In Kevan P, Imperatriz-Fonseca VL (eds) Pollinating bees—the conservation link between agriculture and nature. Ministry of environment, Brasilia, pp 21–28.
Oertli S, Müller A, Dorn S (2005) Ecological and seasonal patterns in the diversity of a species-rich bee assemblage (Hymenoptera: Apoidea: Apiformes). Eur J Entomol 102:53–63.
Oliveira PEM (2008) Fenologia e biologia reprodutiva das espécies de Cerrado. In Sano SM, Almeida SP, Ribeiro JF (eds) Cerrado: ecologia e flora. Embrapa Informação Tecnológica, Brasília, pp 273–290.
Oliveira PE, Gibbs PE (2000) Reproductive biology of woody plants in a cerrado community of Central Brazil. Flora 195:311–329
Oliveira PE, Gibbs PE (2002) Pollination and reproductive biology in cerrado plant communities. In: Oliveira PEz Marquis RJ (ed) The Cerrados of Brazil: ecology and natural history of a Neotropical Savanna. Columbia University, New York, pp 329–347
Oliveira R, Schlindwein C (2009) Searching for a manageable pollinator for acerola orchards: the solitary oil-collecting bee Centris analis (Hymenoptera: Apidae: Centridini). J Econ Entomol 102:265–273. doi:10.1603/029.102.0136
Oliveira-Melazzo AF, Oliveira PE (2012) Cuphea melvilla Lindlay (Lythraceae): uma espécie do cerrado polinizada por beija-flores. Acta Bot Bras 26(2):281–289.
Pacheco Filho AJS, Verola CF, Lima Verde LW, Freitas BM (2015) Bee-flower association in the neotropics: implications to bee conservation and plant pollination. Apidologie 46:530–541
Pais MP, Varanda EM (2010) Arthropod recolonization in the restoration of a semideciduous forest in southeastern Brazil. Neotrop Entomol 39:198–206
Pereira M, Garófalo CA (1996) Aprovisionamento de células por Centris (Hemisiella) vittata Lepeletier. In Garófalo CA et al (eds) Anais do II Encontro sobre Abelhas. Universidade de São Paulo, Ribeirão Preto
Phillips W, Murillo C, Gonzáles Y, Barbeau GB, Ramnanan N, Warnisley D & Lapeyre de Bellaire JD (1994) An overview of the current status of agriculture in Tobago: implications for the future. IICA, Port-of-Spain
Pielou EC (1966) An introduction to mathematical ecology. Wiley, New York
Pina WC, Aguiar CML (2011) Trap-nesting Bees (Hymenoptera: Apidae) in orchards of acerola (Malpighia emarginata) in a semiarid region of Brazil. Sociobiology 58:379–392
Pinheiro M, Sazima M (2007) Visitantes florais e polinizadores de seis espécies arbóreas de Leguminosae melitófilas na Mata Atlântica no sudeste do Brasil. Rev Bras Bioci 5:447–449.
Poderoso JCM, Correia-Oliveira ME, Paz LC, Souza TMS, Vilca FV, Dantas PC, Ribeiro GT (2012) Botanical preferences of africanized bees (Apis mellifera) on the coast and in the atlantic forest of Sergipe, Brazil. Sociobiology 59(1):97–105
R Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.
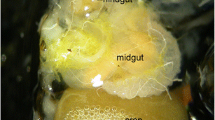
Rabelo LS, Vilhena AMGF, Bastos EMAF, Augusto SC (2012) Larval food sources of Centris (Heterocentris) analis (Fabricius, 1804) (Hymenoptera: Apidae), an oil-collecting bee. J Nat Hist 46:1129–1140. doi:10.1080/00222933.2011.651798
Ramalho M, Rosa JF (2010) Ecologia da interação entre as pequenas flores de quilha de stylosanthes viscosa Sw. (Faboideae) e as grandes abelhas Xylocopa (Neoxylicopa) cearensis Ducke, 1910 (Apoidea, Hymenoptera), em duna tropical. Biota Neotrop 10(3):93–100.
Rao SP, Raju AJS, Victor P (2005) Steady state flowering pattern, temporal dioecism, facultative xenogamy and pollination by insects in Tecoma stans L. (Bignoniaceae). J Natl Taiwan Mus 58(2):109–116
Richards LA, Windsor DM (2007) Seasonal variation of arthropod abundance in gaps and the understory of a lowland moist forest in Panama. J Trop Ecol 23:169–176
Rocha-Filho LC, Silva CI, Gaglianone MC, Augusto SC (2008) Nesting behavior and natural enemies of Epicharis (Epicharis) bicolor Smith 1854 (Hymenoptera Apidae). Trop Zool 21:227–242.
Rosa JF, Ramalho M (2011) The spatial dynamics of diversity in Centridini bees: the abundance of oil-producing flowers as a measure of habitat quality. Apidologie. doi:10.1007/s13592-011-0075-z.
Roubik DW, Moreno JE (1991) Pollen and spores of Barro Colorado Island. Missouri Botanical Gardens Publications, St. Louis
Sazan MS, Queiroz EP, Ferreira-Caliman MJ, Parra-Hinojosa A, Silva CI, Garófalo CA (2014) Manejo dos polinizadores da aceroleira. Holos, Ribeirão Preto
Shannon CEA (1948) Mathematical theory of communication. Bell Syst Tech J 27:379–423
Sigrist MR, Sazima M (2014) Phenology, reproductive biology and diversity of buzzing bees of sympatric Dichorisandra species (Commelinaceae): breeding system and performance of pollinators. Plant Sys Evol 301(3):1005–1015
Silberbauer-Gottsberger I, Gottsberger G (1988). A polinização de plantas do cerrado. Rev Bras Bio 48:651–653.
Silva CI (2009) Distribuição espaço-temporal de recursos florais utilizados por Xylocopa spp. e interação com plantas de cerrado sentido restrito no Triângulo Mineiro. Thesis, Universidade Federal de Uberlândia, Uberlândia.
Silva ALG, Pinheiro MCB (2007) Biologia floral e da polinização de quatro espécies de Eugenia L. (Myrtaceae). Acta Bot Bras 21(1):235–247.
Silva CI, Augusto SC, Sofia SH, Moscheta IS (2007) Bee diversity in Tecoma stans (L.) Kunth (Bignoniaceae): importance for pollination and fruit production. Neotrop Entomol 36:331–341
Silva CI, Arista M, Ortiz PL, Bauermann SG, Evaldt ACP, Oliveira PE (2010) Catálogo polínico: palinologia aplicada em estudos de conservação de abelhas do gênero xylocopa no triângulo mineiro. EDUFU, Uberlândia
Silva NAP, Frizzas MR, Oliveira CM (2011) Seasonality in insect abundance in the “Cerrado” of Goiás State, Brasil. Rev Bras Entomol 55:79–87.
Silva CI, Araújo G, Oliveira PEAM (2012) Distribuição vertical dos sistemas de polinização bióticos em áreas de cerrado sentido restrito no Triângulo Mineiro, MG, Brasil. Acta Bot Bras 26:748–760.
Silva CI, Imperatriz-Fonseca VL, Groppo M et al (2014a) Catálogo polínico das plantas usadas por abelhas no campus da USP de Ribeirão Preto. Holos, Ribeirão Preto
Silva CI, Imperatriz-Fonseca VL, Groppo M, Ferreira-Caliman MJ, Garófalo CA (2014b) Laboratório de palinologia del departamento de biologia de la faculdade de filosofia, ciencias e Letras de Ribeirão Preto, São Paulo, Brasil. Boletín de la Asociación Latinoamerica de Paleobotánica y Palinología 14:185–193.s
Simpson BB, Neff JL, Seigler D (1977) Krameria, free fatty acids and oil-collecting bees. Nature 267:150–151
Thiele, R. (2005). Phenology and nest site preferences of wood-nesting bees in a neotropical lowland rain forest. Stud Neotrop Fauna E 40:39–48.
Tommasi D, Miro A, Higo HA, Winston L (2004) Bee diversity and abundance in an urban setting. Can Entomol 136:851–869
Vieira de Jesus BM, Garófalo CA (2000) Nesting behaviour of Centris (Heterocentris) analis (Fabricius) in southeastern Brasil (Hymenoptera, Apidae, Centridini). Apidologie 31:503–515
Vilhena AMGF, Rabelo LS, Bastos EMAF, Augusto SC (2012) Acerola pollinators in the savanna of central Brazil: temporal variations in oil-collecting bee richness and a mutualistic network. Apidologie 43:51–62
Vinson SB, Williams HJ, Frankie GW, Shrum G (1997) Floral lipid chemistry of Byrsonima crassifolia (Malpigheaceae) and a use of floral lipids by Centris bees (Hymenoptera: Apidae). Biotropica 29:76–83
Vogel S (1974) Ölblumen und ölsammelnde Bienen. Trop Subtrop Pflanzenwelt 7:1–267.
Vogel S (1990) History of the Malpighiaceae in the light of pollination ecology. Mem N Y Bot Gard 55:130–142.
Wcislo WT, Cane JH (1996) Floral resource utilization by solitary bees (Hymenoptera: Apoidea) and exploitation of their stored foods by natural enemies, Ann Rev Entomol 41:257–286.
Westerkamp C (1997) Keel blossoms: bee flowers with adaptations against bees. Flora 192:125–132
Wojcik VA, Frankie GW, Thorp RW, Hernandez J (2008) Seasonality in bees and their floral resource plants at a constructed urban bee habitat in Berkeley, California. J Kansas Entomol Soc 81(1):15–28. doi:10.2317/JKES-701.17.1
Wolda H (1988) Insect seasonality: why?. Annu Rev Ecol Evol Syst 19:1–18.
Wolowski M, Freitas L (2011) Reproduction, pollination and seed predation of Senna multijuga (Fabaceae) in two protected areas in the Brazilian Atlantic forest. Rev Biol Trop 59(4):1669–1678
Wray JC, Neame LA, Elle E (2014) Floral resources, body size, and surrounding landscape influence bee community assemblages in oak-savannah fragments. Ecol Entomol 39:83–93.
Zanette LRS, Martins RP, Ribeiro SP (2005) Effects of urbanization on Neotropical wasp and bee assemblages in a Brazilian metropolis. Landscape Urban Plan 71:105–121.
Zar JH (1999) Biostatistical analysis. Prentice Hall International Editions, New Jersey
Acknowledgements
We thank FAPESP (process #2010/10285-4) and CAPES-PNPD (process #02958/09-0), for financial support, and Research Center on Biodiversity and Computing (BioComp): RCPol-Redes de Catálogos Polínicos online (FDTE process#001505). We thank the editors immensely for the comments that helped to improve this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Isabel Alves dos Santos.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
da Silva, C.I., Hirotsu, C.M., de Suza Pacheco Filho, A.J. et al. Is the maximum reproductive rate of Centris analis (Hymenoptera, Apidae, Centridini) associated with floral resource availability?. Arthropod-Plant Interactions 11, 389–402 (2017). https://doi.org/10.1007/s11829-017-9513-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-017-9513-9