Abstract
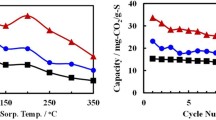
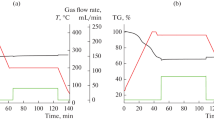
For the development of a dry solid sorbent having quite fast CO2 sorption kinetics in an intermediate temperature range of 245–300 °C to be applicable to a riser-type fluidized bed carbonator, samples of Al2O3-supported MgCO3 (1.2 mmol/g) promoted with different molar amounts of Na2CO3 (1.2, 1.8 mmol/g) and/or NaNO3 (0.6 mmol/g) were prepared by incipient wetness pore volume impregnation. For a reference, an unsupported bulk phase sorbent of NaNO3-Na2Mg(CO3)2 was also prepared. From the sorption reaction using a gas mixture containing CO2 by 2.5–10% at 1 bar for the sorbents after their activation to MgO, Al2O3-supported sorbents were featured by their rapid carbonation kinetics in contrast to the unsupported sorbent showing a quite slow carbonation behavior. The addition of Na2CO3 to the MgCO3/Al2O3 sorbent made MgO species more reactive for the carbonation, bringing about a markedly enhanced kinetic rate and conversion, as compared with the unpromoted MgCO3/Al2O3 sorbent having a small negligible reactivity. The addition of NaNO3 to MgCO3/Al2O3 or to Na2CO3-MgCO3/Al2O3 induced the same promotional effects, but to a lesser magnitude, as observed for the Na2CO3 addition. It was also characteristic for all these MgCO3-based sorbents that initial carbonation conversions with time appeared as sigmoid curves. For the Al2O3-supported sorbent comprised of NaNO3, Na2CO3, and MgCO3 by 0.6, 1.8, and 1.2 mmols, respectively, per gram sorbent, showing the best kinetic performance, a kinetic equation capable of reflecting such sigmoid conversion behavior was established, and its applicability to a riser carbonator was examined throughout a simple model calculation based on the kinetics obtained.
Similar content being viewed by others
References
A. Samanta, A. Zhao, G. K. H. Shimizu, P. Sarkar and R. Gupta, Ind. Eng. Chem. Res., 51, 1438 (2012).
M. Zaman and J. H. Lee, Korean J. Chem. Eng., 30, 1497 (2013).
H. Hayashi, J. Taniuchi, N. Furuyashiki, S. Sugiyama, S. Hirano, N. Shigemoto and T. Nonaka, Ind. Eng. Chem. Res., 37, 185 (1998).
J. C. Abanades, E. J. Anthony, D.Y. Lu, C. Salvador and D. Alvarez, AIChE J., 50, 1614 (2004).
C.-H. Yu, C.-H. Huang and C.-S. Tan, Aerosol Air Quality Res., 12, 745 (2012).
K. Kim, D. Kim, Y.-K. Park and K. S. Lee, Int. J. Greenhous Gas Control, 26, 135 (2014).
M. Iijima, T. Nagayasu, T. Kamijyo and S. Nakatani, Mitsubishi Heavy Industries Technical Review, 48, 26 (2011).
C.-K. Yi, S.-H. Jo, Y. Seo, J.-B. Lee and C.-K. Ryu, Int. J. Greenhouse Gas Control, 1, 31 (2007).
J.-H. Choi, C.-K. Yi and S.-H. Jo, Korean J. Chem. Eng., 28, 1144 (2011).
R. Veneman, Z. S. Li, J.A. Hogendoorn, S.R. A. Kersten and D.W. F. Brilman, Chem. Eng. J., 207–208, 18 (2012).
D.K. Lee, D.Y. Min, H. Seo, N.Y. Kang, W.C. Choi and Y.K. Park, Ind. Eng. Chem. Res., 52, 9323 (2013).
E.R. Monazam, L. J. Shadle, D.C. Miller, H.W. Pennline, D. J. Fauth, J. S. Hoffman and M. L. Gray, AIChE J., 59, 923 (2013).
K. Zhang, X. S. Li, Y. Duan, D. L. King, P. Singh and L. Li, Int. J. Greenhouse Gas Control, 12, 351 (2013).
S. G. Mayorga, S. J. Weigel, T.R. Gaffney and J.R. Brzozowski, US Patent, 6,280,503 B1 (2001).
T. Bauer, D. Laing, U. Kröner and R. Tamme, Int. J. Thermophys., 33, 91 (2012).
R.W. Berg, D. H. Kerridge and P. H. Larsen, J. Chem. Eng. Data, 51, 34 (2006).
C. Zhao, X. Chen and C. Zhao, Ind. Eng. Chem. Res., 51, 14361 (2012).
C. Zhao, X. Chen and C. Zhao, Energy Fuels, 26, 1401 (2012).
G.S. Patience, J. Chaouki, F. Berruti and S.R. Wong, Powder Technol., 72, 31 (1992).
C. J. Geankoplis, Transport processes and separation process principles, 4th Ed., Prentice Hall, U.S.A. (2003).
D. Kunii and O. Levenspiel, Fluidization Engineering, Wiley, N.Y. (1969).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seo, H., Min, D.Y., Kang, N.Y. et al. Development of the Al2O3-supported NaNO3-Na2Mg(CO3)2 sorbent for CO2 capture with facilitated sorption kinetics at intermediate temperatures. Korean J. Chem. Eng. 32, 51–61 (2015). https://doi.org/10.1007/s11814-014-0195-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0195-z