Abstract
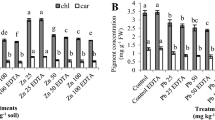
We investigated phytotoxicity in seven plant species exposed to a range of concentrations (0–500 mg·kg−1 soil) of di-n-butyl phthalate (DnBP) or bis (2-ethylhexyl) phthalate (DEHP), two representative phthalate esters (PAEs) nominated by USEPA as priority pollutants and known environmental estrogens. We studied seed germination, root elongation, seedling growth, biomass (fresh weight, FW) and malondialdehyde (MDA) content of shoots and roots of wheat (Triticum aestivum L.), alfalfa (Medicago sativa L.), perennial ryegrass (Lolium perenne), radish (Raphanus sativus L.), cucumber (Cucumis sativus L.), oat (Avena sativa) and onion (Allium cepa L.), together with monitoring of plant pigment content (chlorophyll a, b and carotinoids) in alfalfa, radish and onion shoots. Root elongation, seedling growth and biomass of the test species were generally inhibited by DnBP but not by DEHP, indicating a lower level of phytotoxicity of DEHP than of DnBP. MDA contents of four species were promoted by PAE exposure, but not in alfalfa, ryegrass or onion shoots, indicating lower sensitivity of these three species to PAE pollutants. Plant pigment contents were clearly affected under the stress of both pollutants, implying the potential damage to the photosynthetic system of test plants, mainly by decreasing the content of chlorophyll a and b. Results of DnBP and DEHP phytotoxicity to the primary growth of test plants has provided information for the assessment of their environmental risk in the soil and also forms a basis for the further analysis of their toxic effects over the whole growth period of different plant species.
Similar content being viewed by others
References
Scholz N. Ecotoxicity and biodegradation of phthalate monoesters. Chemosphere, 2003, 53(8): 921–926
Liu W L, Shen C F, Zhang Z, Zhang C B. Distribution of phthalate esters in soil of e-waste recycling sites from Taizhou city in China. Bulletin of Environmental Contamination and Toxicology, 2009, 82(6): 665–667
Zeng F, Cui K Y, Xie Z Y, Wu L N, Luo D L, Chen L X, Lin Y J, Liu M, Sun G X. Distribution of phthalate esters in urban soils of subtropical city, Guangzhou, China. Journal of Hazardous Materials, 2009, 164(2–3): 1171–1178
Vikelsøe J, Thomsen M, Carlsen L. Phthalates and nonylphenols in profiles of differently dressed soils. Science of the Total Environment, 2002, 296(1–3): 105–116
Gibson R, Wang M J, Padgett E, Beck A J. Analysis of 4-nonylphenols, phthalates, and polychlorinated biphenyls in soils and biosolids. Chemosphere, 2005, 61(9): 1336–1344
Hu X X, Han Z H, Liu B Y, Zhang F B, Li F, Wang W H. Distribution of phthalic acid esters in environment and its toxicity. Environmental Science and Management, 2007, 32(1): 37–40
Schowanek D, Carr R, David H, Douben P, Hall J, Kirchmann H, Patria L, Sequi P, Smith S, Webb S. A risk-based methodology for deriving quality standards for organic contaminants in sewage sludge for use in agriculture—Conceptual Framework. Regulatory Toxicology and Pharmacology, 2004, 40(3): 227–251
Chang L W, Meier J R, Smith M K. Application of plant and earthworm bioassays to evaluate remediation of a lead-contaminated soil. Archives of Environmental Contamination and Toxicology, 1997, 32(2): 166–171
An Q, Jin W, Li Y, Xu R W. Influence of plasticizer PAEs to the soil-plant system. Acta Pedologica Sinica, 1999, 2(1): 118–126 (in Chinese)
Yin R, Lin X G, Wang S G, Zhang H Y. Influence of phthalic acid esters in vegetable garden soil on quality of capsicum fruit. Agro-Environmental Protection, 2002, 21(1): 1–4 (in Chinese)
Liao C S, Yen J H, Wang Y S. Growth inhibition in Chinese cabbage (Brassica rapa var. chinensis) growth exposed to di-n-butyl phthalate. Journal of Hazardous Materials, 2009, 163(2–3): 625–631
US Environmental Protection Agency. 1996. Ecological Effects Test Guidelines (OPPTS 850.4200): Seed Germination / Root Elongation Toxicity Test. Available from: http://www.epa.gov/opptsfrs/publications/OPPTSHarmonized/850EcologicalEffectsTestGuidelines/Drafts/850-4200.pdf
Wang X D, Sun C, Gao S X, Wang L S, Shuokui H. Validation of germination rate and root elongation as indicator to assess phytotoxicity with Cucumis sativus. Chemosphere, 2001, 44(8): 1711–1721
Zhang C G, Leung K K, Wong Y S, Tam N F Y. Germination, growthand physiological responses of mangrove plant (Bruguiera gymnorrhiza) to lubricating oil pollution. Environmental and Experimental Botany, 2007, 60(1): 127–136
Lichtenthaler H K, Wellburn A R. Determination of total carotenoids and chlorophyls a and b of leaf extracts in different solvents. Biochemical Society Transactions, 1983, 603(11): 591–592
Wang W, Keturi P H. Comparative seed germination tests using ten plant species for toxicity assessment of metals engraving effluent sample. Water, Air, and Soil Pollution, 1990, 52(3–4): 369–376
Kordan H A. Seed viability and germination: a multi-purpose experimental system. Journal of Biological Education, 1992, 26(4): 247–251
Moore M T, Huggett D B, Huddleston G M III, Rodgers J H Jr, Cooper C M. Herbicide effects on Typha latifolia (Linneaus) germination and root and shoot development. Chemosphere, 1999, 38(15): 3637–3647
Munzuroglu O, Geckil H. Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticum aestivum and Cucumis sativus. Archives of Environmental Contamination and Toxicology, 2002, 43(2): 203–213
Murata M R, Hammes P S, Zharare G E. Effect of solution pH and calcium concentration on germination and early growth of groundnut. Journal of Plant Nutrition, 2003, 26(6): 1247–1262
Lin D H, Xing B S. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environmental Pollution, 2007, 150(2): 243–250
Zheng Z, He P J, Shao L M, Lee D J. Phthalic acid esters in dissolved fractions of landfill leachates. Water Research, 2007, 41(20): 4696–4702
Xu X R, Li X Y. Adsorption behavior of dibutyl phthalate on marine sediments. Marine Pollution Bulletin, 2008, 57(6–12): 430–408
Shiota K, Chou M J, Nishimura H. Embryotoxic effects of di-2-ethylhexyl phthalate (DEHP) and di-n-buty phthalate (DBP) in mice. Environmental Research, 1980, 22(1): 245–253
Defoe D L, Holcombe G W, Hammermeister D E, Biesinger K E. Solubility and toxicity of eight phthalate esters to four aquatic organisms. Environmental Toxicology and Chemistry, 1990, 9(5): 623–636
Staples C A, Adams W J, Parkerton T F, Gorsuch J W, Biddinger G R, Reinert K H. Aquatic toxicity of eighteen phthalate esters. Environmental Toxicology and Chemistry, 1997, 16(5): 875–891
Roslev P, Vorkamp K, Aarup J, Frederiksen K, Nielsen P H. Degradation of phthalate esters in an activated sludge wastewater treatment plant. Water Research, 2007, 41(5): 969–976
Liu Y, Guan Y T, Yang Z H, Cai Z H, Mizuno T, Tsuno H, Zhu W P, Zhang X H. Toxicity of seven phthalate esters to embryonic development of the abalone Haliotis diversicolor supertexta. Ecotoxicology (London, England), 2009, 18(3): 293–303
Santibáñez C, Verdugo C, Ginocchio R. Phytostabilization of copper mine tailings with biosolids: implications for metal uptake and productivity of Lolium perenne. Science of the Total Environment, 2008, 395(1): 1–10
Story K B. Oxidative stress: animal adaptations in nature. Brazilian Journal of Medical and Biological Research, 2006, 29(12): 1715–1733
Jones G J, Nichols P D, Johns B, Smith J D. The effect of mercury and cadmiumon the fatty acid and sterol composition of the marine diatom Asterionella glacialis. Phytochemistry, 1987, 26(5): 1343–1348
Gupta M, Sinha S, Chandra P. Copper-induced toxicity in aquatic macrophyte, Hydrilla verticillata: effect of pH. Ecotoxicology (London, England), 1996, 5(1): 23–33
Singh S, Eapen S, D’Souza S F. Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere, 2006, 62(2): 233–246
Zou T J, Li T X, Zhang X Z, Yu H Y, Luo H B. Lead accumulation and tolerance characteristics of Athyrium wardii (Hook.) as a potential phytostabilizer. Journal of Hazardous Materials, 2011, 186(1): 683–689
Wang S H, Yang Z M, Lu B, Li S Q, Lu Y P. Copper induced stress and antioxidative responses in roots of Brassica juncea L. Botanical Bulletin of Academia Sinica, 2004, 45: 203–212 (in Chinese)
Song N H, Yin X L, Chen G F, Yang H. Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils. Chemosphere, 2007, 68(9): 1779–1787
Fábregas J, Domínguez A, Álvarez D G, Lamela T, Otero A. García álvarez D, Lamela T, Otero A. Induction of astaxanthin accumulation by nitrogen and magnesium deficiencies in Haematococcus pluvialis. Biotechnology Letters, 1998, 20(6): 623–626
Mascher R, Lippmann B, Holzinger S, Bergmann H. Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. Plant Science, 2002, 163(5): 961–969
Lagriffoul A, Mocquot B, Mench M, Vangronsveld J. Cadmium toxicity effects on growth, mineral and chlorophyll contents, and activities of stress related enzymes in young maize plants (Zea mays L.). Plant and Soil, 1998, 200(2): 241–250
Ralph P J. Herbicide toxicity of Halophila ovalis assessed by chlorophyll a fluorescence. Aquatic Botany, 2000, 66(2): 141–152
Sinha S, Saxena R, Singh S. Comparative studies on accumulation of Cr from metal solution and tannery effluent under repeated metal exposure by aquatic plants: its toxic effects. Environmental Monitoring and Assessment, 2002, 80(1): 17–31
Aslan M, Unlü M Y, Türkmen N, Yilmaz Y Z. Sorption of cadmium and effects on growth, protein content, and photosynthetic pigment composition of Nasturtium officinale R. Br. and Mentha aquatica L. Bulletin of Environmental Contamination and Toxicology, 2003, 71(2): 323–329
Vange V, Heuch I, Vandvik V. Do seed mass and family affect germination and juvenile performance in Knautia arvensis? A study using failure-time methods. Acta Oecologica, 2004, 25(3): 169–178
Dolan R W. The effect of seed size and maternal source on individual size in a population of Ludwigia leptocarpa (Onagraceae). American Journal of Botany, 1984, 71(9): 1302–1307
Stanton M L. Seed variation in wild radish: effect of seed size on components of seedling and adult fitness. Ecology, 1984, 65(4): 1105–1112
Winn A A. Ecological and evolutionary consequences of seed size in Prunella vulgaris. Ecology, 1988, 69(5): 1537–1544
Houssard C, Escarré J. The effects of seed weight on growth and competitive ability of Rumex acetosella from two successional oldfields. Oecologia, 1991, 86(2): 236–242
Simons A M, Johnston M O. Variation in seed traits of Lobelia inflata (Campanulaceae): sources and fitness consequences. American Journal of Botany, 2000, 87(1): 124–132
Wulff R D. Seed size variation in Desmodium paniculatum. II. Effects on seedling growth and physiological performance. Journal of Ecology, 1986, 74(1): 99–114
Vaughton G, Ramsey M. Relationships between seed mass, seed nutrients, and seedling growth in Banksia cunninghamii (Proteaceae). International Journal of Plant Sciences, 2001, 162(3): 599–606
George N C, Sands J E. The control of seed germination by moisture as a soil physical property. Australian Journal of Agricultural Research, 1959, 10(5): 628–636
Come D. Obstacles to germination. Monographies de Physiologie Vegetale, 1970, 6: 162
Takemoto B K, Noble R D. Differential sensitivity of duckweeds (Lemnaceae) to sulphite. I. Carbon assimilation and frond replication rate as factors influencing sulphite phytotoxicity under low and high irradiance. New Phytologist, 1986, 103(3): 525–539
Ait B, Audran J C. Response of champenoise grapevine to low temperatures: Changes of shoot and bud proline concentrations in response to low temperatures and correlations with freezing tolerance. Journal of Horticultural Science, 1987, 72(4): 577–582
Blokhina O, Virolainen E, Fagerstedt K V. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany, 2003, 91(2 Spec No): 179–194
Shaharuddin N A, Kawamura F, Sulaiman O, Hashim R. Evaluation on antioxidant activity, antifungal activity and total phenolic of selected commercial Malaysian timbers. In: Proceedings of International Conference on Environmental Research and Technology. Penang Malaysia: Press of the National University of Malaysia, 2008, 970–974
Posmyk M M, Kontek R, Janas K M. Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicology and Environmental Safety, 2009, 72(2): 596–602
Sgherri C, Cosi E, Navari-Izzo F. Phenols and antioxidative status of Raphanus sativus grown in copper excess. Plant Physiology, 2003, 118(1): 21–28
Terry N. Limiting factors in photosynthesis. 1. Use of iron stress to control photochemical capacity in vivo. Plant Physiology, 1980, 65(1): 114–120
Manthey J A, Crowley D E. Leaf and root responses to iron deficiency in avocado. Journal of Plant Nutrition, 1997, 20(1): 683–693
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, T., Teng, Y., Christie, P. et al. Phytotoxicity in seven higher plant species exposed to di-n-butyl phthalate or bis (2-ethylhexyl) phthalate. Front. Environ. Sci. Eng. 9, 259–268 (2015). https://doi.org/10.1007/s11783-014-0652-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-014-0652-2