Abstract
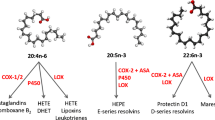
Cyclooxygenase (COX)-2 inhibitors, such as celecoxib, for chronic inflammatory disease are associated with adverse health events, while cis-9, trans-11 (c9t11) conjugated linoleic acid (CLA) is anti-inflammatory without adverse events attributed to pure intake. Mechanistically, celecoxib and c9t11 disrupt the arachidonic acid cascade; however, the equivalency of anti-inflammatory effects between these compounds is unknown. Therefore, to test the hypothesis that 0.5% dietary c9t11 reduces inflammation equivalently to a celecoxib dose intended to treat rheumatoid arthritis (RA; 5 mg/kg bw), arthritic mice received diets containing one of the following supplements: 1% corn oil (CO, w/w), 0.5% c9t11 (>91% purity) +0.5% CO, or 1% CO + 0.5, 5, or 50 mg/kg bw celecoxib, and were assessed for changes in arthritic severity over 6 weeks. Overall, arthritic severity in mice fed c9t11 was reduced (34%, P < 0.01) while celecoxib doses (0.5, 5, 50 mg/kg) reduced arthritic severity (16, 56, 48%, respectively) compared to CO-fed arthritic mice. Linear regression of the celecoxib dose-response showed 0.5% c9t11 (570 mg/kg bw) reduced arthritic severity equivalently to 1.5 mg/kg celecoxib. Interleukin-6 (IL-6) was increased in paws of arthritic mice fed CO compared to shams, but was decreased in arthritic groups fed 0.5% c9t11 and 5 mg/kg celecoxib, compared to arthritic mice fed CO (Ps ≤ 0.05). Additionally, paw and plasma IL-10 levels in arthritic mice were decreased by 5 mg/kg celecoxib, but were unaffected by c9t11 compared to CO. Results suggest dietary c9t11 may be an effective adjunct to COX-2 inhibition for treating chronic inflammation.


Similar content being viewed by others
Abbreviations
- ARA:
-
Arachidonic acid
- AUC:
-
Area under the curve
- c9t11:
-
cis-9, trans-11
- CA:
-
Collagen-induced arthritis
- CAS:
-
Clinical arthritic score
- CLA:
-
Conjugated linoleic acid
- CO:
-
Corn oil
- COX:
-
Cyclooxygenase
- IL:
-
Interleukin
- IMID:
-
Immune-mediated inflammatory disease
- NF-κB:
-
Nuclear factor-kappa B
- nsNSAID:
-
Nonselective non-steroidal anti-inflammatory drug
- PG:
-
Prostaglandin
- PLA:
-
Phospholipase
- PPAR:
-
Peroxisome proliferator-activated receptor
- TXA:
-
Thromboxane
- TNF:
-
Tumor necrosis factor
References
Ward BW, Schiller JS, Goodman RA (2014) Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis 11:E62
Zhou Y, Boudreau DM, Freedman AN (2014) Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general US population. Pharmacoepidemiol Drug Saf 23:43–50
Duffield-Lillico AJ, Boyle JO, Zhou XK, Ghosh A, Butala GS, Subbaramaiah K, Newman RA, Morrow JD, Milne GL, Dannenberg AJ (2009) Levels of prostaglandin E metabolite and leukotriene E(4) are increased in the urine of smokers: evidence that celecoxib shunts arachidonic acid into the 5-lipoxygenase pathway. Cancer Prev Res 2:322–329
Graff J, Skarke C, Klinkhardt U, Watzer B, Harder S, Seyberth H, Geisslinger G, Nüsing RM (2007) Effects of selective COX-2 inhibition on prostanoids and platelet physiology in young healthy volunteers. J Thromb Haemost 5:2376–2385
Page TH, Turner JJO, Brown AC, Timms EM, Inglis JJ, Brennan FM, Foxwell BMJ, Ray KP, Feldmann M (2010) Nonsteroidal anti-inflammatory drugs increase TNF production in rheumatoid synovial membrane cultures and whole blood. J Immunol 185:3694–3701
Gomez PF, Pillinger MH, Attur M, Marjanovic N, Dave M, Park J, Bingham CO, Al-Mussawir H, Abramson SB (2005) Resolution of inflammation: prostaglandin E2 dissociates nuclear trafficking of individual NF-kappaB subunits (p65, p50) in stimulated rheumatoid synovial fibroblasts. J Immunol 175:6924–6930
Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T (1994) Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med 180:2365–2370
Cheon H, Rho YH, Choi SJ, Lee YH, Song GG, Sohn J, Won NH, Ji JD (2006) Prostaglandin E2 augments IL-10 signaling and function. J Immunol 177:1092–1100
Aryaeian N, Shahram F, Djalali M, Eshragian MR, Djazayeri A, Sarrafnejad A, Salimzadeh A, Naderi N, Maryam C (2009) Effect of conjugated linoleic acids, vitamin E and their combination on the clinical outcome of Iranian adults with active rheumatoid arthritis. Int J Rheum Dis 12:20–28
Huebner SM, Campbell JP, Butz DE, Fulmer TG, Gendron-Fitzpatrick A, Cook ME (2010) Individual isomers of conjugated linoleic acid reduce inflammation associated with established collagen-induced arthritis in DBA/1 mice. J Nutr 140:1454–1461
Huebner SM, Olson JM, Campbell JP, Bishop JW, Crump PM, Cook ME (2016) Low dietary c9t11-conjugated linoleic acid intake from dairy fat or supplements reduces inflammation in collagen-induced arthritis. Lipids 51:807–819
Stachowska E, Dziedziejko V, Safranow K, Gutowska I, Adler G, Ciechanowicz A, Machaliński B, Chlubek D (2007) Inhibition of phospholipase A(2) activity by conjugated linoleic acids in human macrophages. Eur J Nutr 46:28–33
Huebner SM, Olson JM, Campbell JP, Bishop JW, Crump PM, Cook ME (2014) Dietary trans-10, cis-12 CLA reduces murine collagen-induced arthritis in a dose-dependent manner. J Nutr 144(2):177–184
Butz DE, Li G, Huebner SM, Cook ME (2007) A mechanistic approach to understanding conjugated linoleic acid’s role in inflammation using murine models of rheumatoid arthritis. Am J Physiol Regul Integr Comp Physiol 293:R669–R676
Darwish HA, Arab HH, Abdelsalam RM (2014) Chrysin alleviates testicular dysfunction in adjuvant arthritic rats via suppression of inflammation and apoptosis: comparison with celecoxib. Toxicol Appl Pharmacol 279:129–140
Khayyal MT, El-Ghazaly MA, Abdallah DM, Okpanyi SN, Kelber O, Weiser D (2005) Mechanisms involved in the anti-inflammatory effect of a standardized willow bark extract. Drug Res 55:677–687
Turpeinen AM, Mutanen M, Aro A, Salminen I, Basu S, Palmquist DL, Griinari JM (2002) Bioconversion of vaccenic acid to conjugated linoleic acid in humans. Am J Clin Nutr 76:504–510
Shingfield KJ, Reynolds CK, Hervas G (2006) Examination of the persistency of milk fatty acid composition responses to fish oil and sunflower oil in the diet of dairy cows. J Dairy Sci 89:714–732
Jaudszus A, Krokowski M, Mo P, Darcan Y, Avagyan A (2008) Cis-9, trans-11-conjugated linoleic acid inhibits allergic sensitization and airway inflammation via a pparg-related mechanism in mice. J Nutr 138:1336–1342
Bassaganya-Riera J, Reynolds K, Martino-Catt S, Cui Y, Hennighausen L, Gonzalez F, Rohrer J, Benninghoff AU, Hontecillas R (2004) Activation of PPAR γ and δ by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology 127:777–791
Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK (1998) The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391:79–82
Ringseis R, Müller A, Herter C, Gahler S, Steinhart H, Eder K (2006) CLA isomers inhibit TNFalpha-induced eicosanoid release from human vascular smooth muscle cells via a PPARgamma ligand-like action. Biochim Biophys Acta 1760:290–300
Patel S, Vetale S, Teli P, Mistry R, Chiplunkar S (2012) IL-10 production in non-small cell lung carcinoma patients is regulated by ERK, P38 and COX-2. J Cell Mol Med 16:531–544
Negi AK, Renuka Bhatnagar A, Agnihotri N (2016) Celecoxib and fish oil: a combination strategy for decreased inflammatory mediators in early stages of experimental mammary cancer. Inflammopharmacology 24:11–22
Henningsson L, Eneljung T, Jirholt P, Tengvall S, Lidberg U, van den Berg WB, van de Loo FA, Gjertsson I (2012) Disease-dependent local il-10 production ameliorates collagen induced arthritis in mice. PLoS One 7:e49731
Shah N, Kammermeier J, Elawad M, Glocker E-O (2012) Interleukin-10 and interleukin-10–receptor defects in inflammatory bowel disease. Curr Allergy Asthma Rep 12:373–379
Kole A, Maloy KJ (2014) Control of Intestinal Inflammation by Interleukin-10. In: Fillatreau S, O’Garra A (eds) Interleukin-10 heal. Springer, Berlin, pp 19–38
Johansson A, Hansson A-S, Nandakumar KS, Bäcklund J, Holmdahl R (2001) IL-10-deficient B10.Q mice develop more severe collagen-induced arthritis, but are protected from arthritis induced with anti-type II collagen antibodies. J Immunol 167:3505–3512
Finnegan A, Kaplan CD, Cao Y, Eibel H, Glant TT, Zhang J (2003) Collagen-induced arthritis is exacerbated in IL-10-deficient mice. Arthritis Res Ther 5:R18
Sigthorsson G, Simpson RJ, Walley M, Anthony A, Foster R, Hotz-Behoftsitz C, Palizban A, Pombo J, Watts J, Morham SG, Bjarnason I (2002) COX-1 and 2, intestinal integrity, and pathogenesis of nonsteroidal anti-inflammatory drug enteropathy in mice. Gastroenterology 122:1913–1923
Mallen SR, Essex MN, Zhang R (2011) Gastrointestinal tolerability of NSAIDs in elderly patients: a pooled analysis of 21 randomized clinical trials with celecoxib and nonselective NSAIDs. Curr Med Res Opin 27:1359–1366
Park Y (1996) Regulation of energy metabolism and the catabolic effects of immune stimulation by conjugated linoleic acid. University of Wisconsin-Madison, ProQuest Dissertations Publishing, 9610578
McCormack PL (2011) Celecoxib: a review of its use for symptomatic relief in the treatment of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis. Drugs 71:2457–2489
Gong L, Thorn CF, Bertagnolli MM, Grosser T, Altman RB, Klein TE (2012) Celecoxib pathways: pharmacokinetics and pharmacodynamics. Pharmacogen Genom 22:310–318
Brand DD, Latham KA, Rosloniec EF (2007) Collagen-induced arthritis. Nat Protoc 2:1269–1275
Hawkins P, Armstrong R, Boden T, Garside P, Knight K, Lilley E, Seed M, Wilkinson M, Williams RO (2015) Applying refinement to the use of mice and rats in rheumatoid arthritis research. Inflammopharmacology 23:131–150
Liu X, Zhou H, Huang X, Cui J, Long T, Xu Y, Liu H, Yu R, Zhao R, Luo G, Huang A, Liang JG, Liang P (2016) A broad blockade of signaling from the il-20 family of cytokines potently attenuates collagen-induced arthritis. J Immunol 197:3029–3037
Acknowledgements
The authors express their gratitude toward Shane Huebner for technical assistance. The authors thank Dawn Irish, Angel Guterriez-Velin, and Terry Jobsis for their assistance with animal husbandry. The authors would like to thank Peter Crump for his assistance with statistical analyses. This research was partially supported by a USDA Hatch Project Grant (MSN130802), patent royalties from the Wisconsin Alumni Research Foundation (WARF), and the College of Agricultural and Life Sciences at the University of Wisconsin-Madison.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest.
Additional information
This project was partially supported by a USDA Hatch project grants (MSN130802), patent royalties from the Wisconsin Alumni Research Foundation (WARF), and the College of Agricultural and Life Sciences at the University of Wisconsin-Madison.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Olson, J.M., Haas, A.W., Lor, J. et al. A Comparison of the Anti-Inflammatory Effects of Cis-9, Trans-11 Conjugated Linoleic Acid to Celecoxib in the Collagen-Induced Arthritis Model. Lipids 52, 151–159 (2017). https://doi.org/10.1007/s11745-016-4228-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-016-4228-8