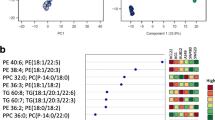
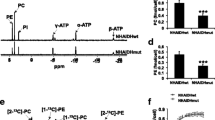
Abstract
Glucose and glutamine are essential energy metabolites for brain tumor growth and survival under both normoxic and hypoxic conditions. Both metabolites can contribute their carbons to lipid biosynthesis. We used uniformly labeled [14C]-U-d-glucose and [14C]-U-l-glutamine to examine the profile of de novo lipid biosynthesis in the VM-M3 murine glioblastoma cells. The major lipids synthesized included phosphatidylcholine (PtdCho), phosphatidylethanolamine (EtnGpl), phosphatidylinositol (PtdIns), phosphatidylserine (PtdSer), sphingomyelin (CerPCho), bis(monoacylglycero)phosphate (BMP)/phosphatidic acid (PtdOH), cholesterol (C), cardiolipin (Ptd2Gro), and gangliosides. Endogenous lipid synthesis, using either glucose or glutamine, was greater in media without fetal bovine serum (FBS) than in media containing 10 % FBS under normoxia. De novo lipid synthesis was greater using glucose carbons than glutamine carbons under normoxia. The reverse was observed for most lipids under hypoxia suggesting an attenuation of glucose entering the TCA cycle. Lactate was produced largely from glucose carbons with minimal lactate derived from glutamine under either normoxia or hypoxia. Accumulation of triacylglycerols (TAG), containing mostly saturated and mono-unsaturated fatty acids, was observed under hypoxia using carbons from either glucose or glutamine. The data show that the incorporation of labeled glucose and glutamine into most synthesized lipids was dependent on the type of growth environment, and that the VM-M3 glioblastoma cells could acquire lipids, especially cholesterol, from the external environment for growth and proliferation.









Similar content being viewed by others
Abbreviations
- AA:
-
Antimycin A
- ACLY:
-
ATP citrate lyase
- ACC:
-
Acetyl CoA carboxylase
- BMP:
-
Bis(monoacylglycero)phosphate
- C:
-
Cholesterol
- Cer:
-
Ceramide
- Ptd2Gro:
-
Cardiolipin
- FASN:
-
Fatty acid synthase
- FBS:
-
Fetal bovine serum
- GLC:
-
Gas liquid chromatography
- HPTLC:
-
High performance thin-layer chromatography
- lysoPtdCho:
-
Lysophosphatidylcholine
- PtdOH:
-
Phosphatidic acid
- PtdCho:
-
Phosphatidylcholine
- EtnGpl:
-
Phosphatidylethanolamine
- PtdGro:
-
Phosphatidylglycerol
- PtdIns:
-
Phosphatidylinositol
- PtdSer:
-
Phosphatidylserine
- CerPCho:
-
Sphingomyelin
- TAG:
-
Triacylglycerol
References
Seyfried TN, Flores R, Poff AM, D’Agostino DP, Mukherjee P (2014) Metabolic therapy: a new paradigm for managing malignant brain cancer. Cancer Lett 356:289–300
Wen PY, Kesari S (2008) Malignant gliomas in adults. The New England journal of medicine 359:492–507
Portais JC, Voisin P, Merle M, Canioni P (1996) Glucose and glutamine metabolism in C6 glioma cells studied by carbon 13 NMR. Biochimie 78:155–164
DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB (2007) Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci 104:19345–19350
Wolf A, Agnihotri S, Guha A (2010) Targeting metabolic remodeling in glioblastoma multiforme. Oncotarget 1:552–562
Maher EA, Marin-Valencia I, Bachoo RM, Mashimo T, Raisanen J, Hatanpaa KJ, Jindal A, Jeffrey FM, Choi C, Madden C, Mathews D, Pascual JM, Mickey BE, Malloy CR, DeBerardinis RJ (2012) Metabolism of [U-13 C]glucose in human brain tumors in vivo. NMR Biomed 25:1234–1244
Qu W, Oya S, Lieberman BP, Ploessl K, Wang L, Wise DR, Divgi CR, Chodosh LA, Thompson CB, Kung HF (2012) Preparation and characterization of L-[5-11C]-glutamine for metabolic imaging of tumors. J Nucl Med Off Publ, Soc Nucl Med 53:98–105
Gambhir SS (2002) Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer 2:683–693
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Lunt SY, Vander Heiden MG (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27:441–464
Park JB, Lee CS, Jang JH, Ghim J, Kim YJ, You S, Hwang D, Suh PG, Ryu SH (2012) Phospholipase signalling networks in cancer. Nat Rev Cancer 12:782–792
Mills GB, Moolenaar WH (2003) The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 3:582–591
Karmali RA (1986) Eicosanoids and cancer. Prog Clin Biol Res 222:687–697
Wang D, Wang H, Brown J, Daikoku T, Ning W, Shi Q, Richmond A, Strieter R, Dey SK, DuBois RN (2006) CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med 203:941–951
Rolin J, Maghazachi AA (2011) Effects of lysophospholipids on tumor microenvironment. Cancer Microenviron Off J Int Cancer Microenviron Soc 4:393–403
Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, Thompson CB, Rabinowitz JD (2013) Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci 110:8882–8887
Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, Lane TF, Hla T (2004) Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci 101:591–596
Clendening JW, Pandyra A, Boutros PC, El Ghamrasni S, Khosravi F, Trentin GA, Martirosyan A, Hakem A, Hakem R, Jurisica I, Penn LZ (2010) Dysregulation of the mevalonate pathway promotes transformation. Proc Natl Acad Sci 107:15051–15056
Medes G, Thomas A, Weinhouse S (1953) Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res 13:27–29
Ookhtens M, Kannan R, Lyon I, Baker N (1984) Liver and adipose tissue contributions to newly formed fatty acids in an ascites tumor. Am J Physiol 247:R146–R153
Yates AJ, Thompson DK, Boesel CP, Albrightson C, Hart RW (1979) Lipid composition of human neural tumors. J Lipid Res 20:428–436
Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ (2012) Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481:385–388
Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden MG, Iliopoulos O, Stephanopoulos G (2012) Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481:380–384
Scott DA, Richardson AD, Filipp FV, Knutzen CA, Chiang GG, Ronai ZA, Osterman AL, Smith JW (2011) Comparative metabolic flux profiling of melanoma cell lines: beyond the Warburg effect. J Biol Chem 286:42626–42634
Pizer ES, Chrest FJ, DiGiuseppe JA, Han WF (1998) Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res 58:4611–4615
Pizer ES, Jackisch C, Wood FD, Pasternack GR, Davidson NE, Kuhajda FP (1996) Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res 56:2745–2747
Hochachka PW, Rupert JL, Goldenberg L, Gleave M, Kozlowski P (2002) Going malignant: the hypoxia-cancer connection in the prostate. BioEssay 24:749–757
Holleran AL, Briscoe DA, Fiskum G, Kelleher JK (1995) Glutamine metabolism in AS-30D hepatoma cells. Evidence for its conversion into lipids via reductive carboxylation. Mol Cell Biochem 152:95–101
Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniels VW, Machiels J, Vanderhoydonc F, Smans K, Waelkens E, Verhoeven G, Swinnen JV (2010) De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res 70:8117–8126
Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA, Kuhajda FP (2000) Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res 60:213–218
Abraham S, Chaikoff IL (1965) Metabolism of Barrett mammary adenocarcinoma: glucose utilization, fatty acid synthesis, and terminal oxidative patterns and glutamine synthesis. Cancer Res 25:647–655
Santos CR, Schulze A (2012) Lipid metabolism in cancer. FEBS J 279:2610–2623
Beaty NB, Lane MD (1983) Kinetics of activation of acetyl-CoA carboxylase by citrate. Relationship to the rate of polymerization of the enzyme. J Biol Chem 258:13043–13050
Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB (2005) ATP citrate lyase is an important component of cell growth and transformation. Oncogene 24:6314–6322
Smith S (1994) The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. FASEB J 8:1248–1259
Bloch K (1965) The biological synthesis of cholesterol. Science 150:19–28
Cairns RA, Harris I, McCracken S, Mak TW (2011) Cancer cell metabolism. Cold Spring Harb Symp Quant Biol 76:299–311
DeBerardinis RJ, Thompson CB (2012) Cellular metabolism and disease: what do metabolic outliers teach us? Cell 148:1132–1144
Owen OE, Kalhan SC, Hanson RW (2002) The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 277:30409–30412
Reitzer LJ, Wice BM, Kennell D (1979) Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem 254:2669–2676
Kovacevic Z, Morris HP (1972) The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res 32:326–333
Weljie AM, Jirik FR (2011) Hypoxia-induced metabolic shifts in cancer cells: moving beyond the Warburg effect. Int J Biochem Cell Biol 43:981–989
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7:763–777
Stoll LL, Spector AA (1984) Changes in serum influence the fatty acid composition of established cell lines. In vitro 20:732–738
Rothblat GH, Arbogast LY, Ouellette L, Howard BV (1976) Preparation of delipidized serum protein for use in cell culture systems. In vitro 12:554–557
Huysentruyt LC, Mukherjee P, Banerjee D, Shelton LM, Seyfried TN (2008) Metastatic cancer cells with macrophage properties: evidence from a new murine tumor model. Int J Cancer 123:73–84
Shelton LM, Mukherjee P, Huysentruyt LC, Urits I, Rosenberg JA, Seyfried TN (2010) A novel pre-clinical in vivo mouse model for malignant brain tumor growth and invasion. J Neurooncol 99:165–176
Huysentruyt LC, Akgoc Z, Seyfried TN (2011) Hypothesis: are neoplastic macrophages/microglia present in glioblastoma multiforme? ASN neuro 3:AN20110011
Seyfried TN, Glaser GH, Yu RK (1978) Cerebral, cerebellar, and brain stem gangliosides in mice susceptible to audiogenic seizures. J Neurochem 31:21–27
Baek RC, Kasperzyk JL, Platt FM, Seyfried TN (2004) N-butyldeoxygalactonojirimycin reduces brain ganglioside and GM2 content in neonatal sandhoff disease mice. J Neurochem 90(Suppl 1):89
Macala LJ, Yu RK, Ando S (1983) Analysis of brain lipids by high performance thin-layer chromatography and densitometry. J Lipid Res 24:1243–1250
Kasperzyk JL, d’Azzo A, Platt FM, Alroy J, Seyfried TN (2005) Substrate reduction reduces gangliosides in postnatal cerebrum-brainstem and cerebellum in GM1 gangliosidosis mice. J Lipid Res 46:744–751
Kasperzyk JL, El-Abbadi MM, Hauser EC, D’Azzo A, Platt FM, Seyfried TN (2004) N-butyldeoxygalactonojirimycin reduces neonatal brain ganglioside content in a mouse model of GM1 gangliosidosis. J Neurochem 89:645–653
Yuneva M (2008) Finding an “Achilles’ heel” of cancer: the role of glucose and glutamine metabolism in the survival of transformed cells. Cell Cycle 7:2083–2089
Teicher BA, Linehan WM, Helman LJ (2012) Targeting cancer metabolism. Clin Cancer Res Off J Am Assoc Cancer Res 18:5537–5545
Vander Heiden MG (2011) Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 10:671–684
Dang CV, Hamaker M, Sun P, Le A, Gao P (2011) Therapeutic targeting of cancer cell metabolism. J Mol Med 89:205–212
Rodriguez-Enriquez S, Marin-Hernandez A, Gallardo-Perez JC, Carreno-Fuentes L, Moreno-Sanchez R (2009) Targeting of cancer energy metabolism. Mol Nutr Food Res 53:29–48
Piva TJ, McEvoy-Bowe E (1998) Oxidation of glutamine in HeLa cells: role and control of truncated TCA cycles in tumour mitochondria. J Cell Biochem 68:213–225
Souba WW (1993) Glutamine and cancer. Ann Surg 218:715–728
Moreadith RW, Lehninger AL (1984) The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P) + -dependent malic enzyme. J Biol Chem 259:6215–6221
Lu W, Pelicano H, Huang P (2010) Cancer metabolism: is glutamine sweeter than glucose? Cancer Cell 18:199–200
Meng M, Chen S, Lao T, Liang D, Sang N (2010) Nitrogen anabolism underlies the importance of glutaminolysis in proliferating cells. Cell Cycle 9:3921–3932
Zhang F, Du G (2012) Dysregulated lipid metabolism in cancer. World J Biol Chem 3:167–174
Vazquez-Martin A, Colomer R, Brunet J, Lupu R, Menendez JA (2008) Overexpression of fatty acid synthase gene activates HER1/HER2 tyrosine kinase receptors in human breast epithelial cells. Cell Prolif 41:59–85
Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB (2005) ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 8:311–321
Ecsedy JA, Holthaus KA, Yohe HC, Seyfried TN (1999) Expression of mouse sialic acid on gangliosides of a human glioma grown as a xenograft in SCID mice. J Neurochem 73:254–259
Louie SM, Roberts LS, Mulvihill MM, Luo K, Nomura DK (2013) Cancer cells incorporate and remodel exogenous palmitate into structural and oncogenic signaling lipids. Biochim Biophys Acta 1831:1566–1572
Kuemmerle NB, Rysman E, Lombardo PS, Flanagan AJ, Lipe BC, Wells WA, Pettus JR, Froehlich HM, Memoli VA, Morganelli PM, Swinnen JV, Timmerman LA, Chaychi L, Fricano CJ, Eisenberg BL, Coleman WB, Kinlaw WB (2011) Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther 10:427–436
Filipp FV, Scott DA, Ronai ZA, Osterman AL, Smith JW (2012) Reverse TCA cycle flux through isocitrate dehydrogenases 1 and 2 is required for lipogenesis in hypoxic melanoma cells. Pigment Cell Melanoma Res 25:375–383
Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB (2011) Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci 108:19611–19616
van den Heuvel AP, Jing J, Wooster RF, Bachman KE (2012) Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol Ther 13:1185–1194
Fan J, Kamphorst JJ, Mathew R, Chung MK, White E, Shlomi T, Rabinowitz JD (2013) Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol Syst Biol 9:712
McGuirk S, Gravel SP, Deblois G, Papadopoli DJ, Faubert B, Wegner A, Hiller K, Avizonis D, Akavia UD, Jones RG, Giguere V, St-Pierre J (2013) PGC-1alpha supports glutamine metabolism in breast cancer. Cancer Metab 1:22
Semenza GL (2007) HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr 39:231–234
Semenza GL, Artemov D, Bedi A, Bhujwalla Z, Chiles K, Feldser D, Laughner E, Ravi R, Simons J, Taghavi P, Zhong H (2001) The metabolism of tumours’: 70 years later. Novartis Found Symp 240:251–260 (discussion 260–254 )
Dang CV, Semenza GL (1999) Oncogenic alterations of metabolism. Trends Biochem Sci 24:68–72
Mylonis I, Sembongi H, Befani C, Liakos P, Siniossoglou S, Simos G (2012) Hypoxia causes triglyceride accumulation by HIF-1-mediated stimulation of lipin 1 expression. J Cell Sci 125:3485–3493
Brose SA, Marquardt AL, Golovko MY (2014) Fatty acid biosynthesis from glutamate and glutamine is specifically induced in neuronal cells under hypoxia. J Neurochem 129:400–412
Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, Pinnick KE, Wigfield S, Buffa FM, Li JL, Zhang Q, Wakelam MJ, Karpe F, Schulze A, Harris AL (2014) Fatty acid uptake and lipid storage induced by HIF-1alpha contribute to cell growth and survival after hypoxia-reoxygenation. Cell reports 9:349–365
Liu K, Czaja MJ (2013) Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ 20:3–11
Singh R, Cuervo AM (2012) Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol 2012:282041
Tugnoli V, Tosi MR, Tinti A, Trinchero A, Bottura G, Fini G (2001) Characterization of lipids from human brain tissues by multinuclear magnetic resonance spectroscopy. Biopolymers 62:297–306
Opstad KS, Ladroue C, Bell BA, Griffiths JR, Howe FA (2007) Linear discriminant analysis of brain tumour (1)H MR spectra: a comparison of classification using whole spectra versus metabolite quantification. NMR Biomed 20:763–770
Nguyen AD, McDonald JG, Bruick RK, DeBose-Boyd RA (2007) Hypoxia stimulates degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase through accumulation of lanosterol and hypoxia-inducible factor-mediated induction of insigs. J Biol Chem 282:27436–27446
Summons RE, Bradley AS, Jahnke LL, Waldbauer JR (2006) Steroids, triterpenoids and molecular oxygen. Philos Trans R Soc Lond B Biol Sci 361:951–968
Yin J, Hashimoto A, Izawa M, Miyazaki K, Chen GY, Takematsu H, Kozutsumi Y, Suzuki A, Furuhata K, Cheng FL, Lin CH, Sato C, Kitajima K, Kannagi R (2006) Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res 66:2937–2945
Bouterfa HL, Sattelmeyer V, Czub S, Vordermark D, Roosen K, Tonn JC (2000) Inhibition of Ras farnesylation by lovastatin leads to downregulation of proliferation and migration in primary cultured human glioblastoma cells. Anticancer Res 20:2761–2771
Bifulco M (2005) Role of the isoprenoid pathway in ras transforming activity, cytoskeleton organization, cell proliferation and apoptosis. Life Sci 77:1740–1749
Zhong WB, Liang YC, Wang CY, Chang TC, Lee WS (2005) Lovastatin suppresses invasiveness of anaplastic thyroid cancer cells by inhibiting Rho geranylgeranylation and RhoA/ROCK signaling. Endocr Relat Cancer 12:615–629
Thibault A, Samid D, Tompkins AC, Figg WD, Cooper MR, Hohl RJ, Trepel J, Liang B, Patronas N, Venzon DJ, Reed E, Myers CE (1996) Phase I study of lovastatin, an inhibitor of the mevalonate pathway, in patients with cancer. Clin Cancer Off J Am Assoc Cancer Res 2:483–491
Larner J, Jane J, Laws E, Packer R, Myers C, Shaffrey M (1998) A phase I-II trial of lovastatin for anaplastic astrocytoma and glioblastoma multiforme. Am J Clin Oncol 21:579–583
Kim WS, Kim MM, Choi HJ, Yoon SS, Lee MH, Park K, Park CH, Kang WK (2001) Phase II study of high-dose lovastatin in patients with advanced gastric adenocarcinoma. Invest New Drugs 19:81–83
Lersch C, Schmelz R, Erdmann J, Hollweck R, Schulte-Frohlinde E, Eckel F, Nader M, Schusdziarra V (2004) Treatment of HCC with pravastatin, octreotide, or gemcitabine—a critical evaluation. Hepatogastroenterology 51:1099–1103
Baek RC, Kasperzyk JL, Platt FM, Seyfried TN (2008) N-butyldeoxygalactonojirimycin reduces brain ganglioside and GM2 content in neonatal sandhoff disease mice. Neurochem Int 52:1125–1133
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ta, N.L., Seyfried, T.N. Influence of Serum and Hypoxia on Incorporation of [14C]-d-Glucose or [14C]-l-Glutamine into Lipids and Lactate in Murine Glioblastoma Cells. Lipids 50, 1167–1184 (2015). https://doi.org/10.1007/s11745-015-4075-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-4075-z
Keywords
- Fatty acid analysis
- Analytical techniques GLC (GC) (gas-liquid chromatography)
- Analytical techniques, radiotracer
- Analytical techniques, thin layer chromatography
- Analytical techniques, triglyceride analysis
- Analytical techniques, lipid biochemistry
- General area, lipogenesis
- Energy metabolism
- Glioblastoma
- Hypoxia