Abstract
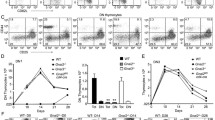
Glycerol 3-phosphate acyltransferase-1 (GPAT-1) catalyzes the initial and rate-limiting step in de novo glycerophospholipid and triacylglycerol (TAG) biosynthesis. We have previously shown that peripheral T cell proliferation and cytokine production is altered in GPAT-1 gene-ablated (KO) mice. This finding is important in light of the reduction in GPAT-1 activity associated with aged T cells. To determine if the mechanism for altered peripheral T cell function is linked to altered T cell development, we assessed thymic function in 3, 6 and 16-week old GPAT-1 KO compared to wild type (WT) mice. At 16 weeks of age, there was a significant reduction in thymic T cell production in KO compared to WT mice but not at 6 weeks of age. The reduced thymic T cell production was associated with altered thymic development as confirmed by increased numbers of double-negative (DN) thymocytes and a significant reduction in the double positive (DP) thymocytes suggesting a developmental block at the DN stage. This change was accompanied by an increase in the single positive CD4 subset. These changes were associated with reduced glycerophospholipid mass while thymic cortex and medulla architecture was not altered by GPAT-1 KO. Taken together, these data suggest that GPAT-1 deletion is capable of reducing the number of new T cells produced via alterations in membrane receptor function rather than by causing deleterious changes within the thymic microenvironment explaining in part the observed alterations in peripheral T cell function.








Similar content being viewed by others
Abbreviations
- Ptd2Gro:
-
Cardiolipin
- CPT-1:
-
Carnitine Palmitoyl Transferase-1
- AMPK:
-
5′Adenosine monophosphate-activated protein kinase
- DN:
-
Double negative
- DP:
-
Double positive
- GPAT-1:
-
Glycerol-3-phosphate acyltransferase-1
- NaHCO3 :
-
Sodium bicarbonate
- NaN3 :
-
Sodium Azide
- pAMPK:
-
Phospho-5′-adenosine monophosphate-activated protein kinase
- PtdOH:
-
Phosphatidic acid
- PBS:
-
Phosphate buffered saline
- ChoGpl:
-
Choline glycerophospholipids
- EtnGpl:
-
Ethanolamine glycerophospholipids
- PtdIns:
-
Phosphatidylinositol
- PtdSer:
-
Phosphatidylserine
- CerPCho:
-
Sphingomyelin
- SP:
-
Single positive
References
Mathis D, Shoelson SE (2011) Immunometabolism: an emerging frontier. Nat Rev Immunol 11:81
Powell JD, Delgoffe GM (2010) The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity 33:301–311
Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang L-S, Jones RG, Choi Y (2009) Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460:103–107
Wendel AA, Lewin TM, Coleman RA (2009) Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis. Biochim Biophys Acta 1791:501–506
Takeuchi K, Reue K (2009) Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol: Endocrin 296:E1195–E1209
Gonzalez-Baro MR, Lewin TM, Coleman RA (2007) Regulation of triglyceride metabolism II. function of mitochondrial GPAT1 in the regulation of triacylglycerol biosynthesis and insulin action. Am J Physiol Gastrointest Liver Physiol 292:G1195–G1199
Collison LW, Murphy EJ, Jolly CA (2008) Glycerol-3-phosphate acyltransferase-1 regulates murine T-lymphocyte proliferation and cytokine production. Am J Physiol: Cell Physiol 295:C1543–C1549
Stapleton CM, Mashek DG, Wang S, Nagle CA, Cline GW, Thuillier P, Leesnitzer LM, Li LO, Stimmel JB, Shulman GI, Coleman RA (2011) Lysophosphatidic acid activates peroxisome proliferator activated receptor-γ in CHO cells that over-express glycerol 3-phosphate acyltransferase-1. PLoS ONE 6:e18932
Zhang C, Wendel AA, Keogh MR, Harris TE, Chen J, Coleman RA (2012) Glycerolipid signals alter mTOR complex 2 (mTORC2) to diminish insulin signaling. Proc Natl Acad Sci 109:1667–1672
Collison LW, Jolly CA (2006) Phosphorylation regulates mitochondrial glycerol-3-phosphate-1 acyltransferase activity in T-lymphocytes. Biochim Biophys Acta 1761:129–139
Karlsson EA, Wang S, Shi Q, Coleman RA, Beck MA (2009) Glycerol-3-phosphate acyltransferase 1 is essential for the immune response to infection with Coxsackie virus B3 in Mice. J Nutr 139(4):779–783
Collison LW, Kannan L, Onorato TM, Knudsen J, Haldar D, Jolly CA (2005) Aging reduces glycerol-3-phosphate acyltransferase activity in activated rat splenic T-lymphocytes. Biochim Biophys Acta 1687:164–172
Lim BO, Jolly CA, Zaman K, Fernandes G (2000) Dietary (n-6) and (n-3) fatty acids and energy restriction modulate mesenteric lymph node lymphocyte function in autoimmune-prone (NZB × NZW) F1 mice. J Nutr 130:1657–1664
Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA (1998) Changes in thymic function with age and during the treatment of HIV infection. Nature 396:690–695
Xu Z, George C, Jolly CA (2004) CD28 activation does not down-regulate Cbl-b expression in aged rat T-lymphocytes. Mech Ag Develop 125:595–602
Klug DB, Carter C, Gimenez-Conti IB, Richie ER (2002) Cutting edge: thymocyte-independent and thymocyte-dependent phases of epithelial patterning in the fetal thymus. J Immunol 169:2842–2845
Yang H, Youm Y-H, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD (2009) Obesity accelerates thymic aging. Blood 114:3803–3812
Yang H, Youm Y-H, Dixit VD (2009) Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J Immunol 183:3040–3052
Littman DR (1999) The regulation of CD4 and CD8 coreceptor gene expression during T cell development. Ann Rev Immunol 17:523–554
Finlay D, Cantrell DA (2011) Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol 11:109–117
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Gulvady, A.A., Murphy, E.J., Ciolino, H.P. et al. Glycerol-3-Phosphate Acyltransferase-1 Gene Ablation Results in Altered Thymocyte Lipid Content and Reduces Thymic T Cell Production in Mice. Lipids 48, 3–12 (2013). https://doi.org/10.1007/s11745-012-3741-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-012-3741-7