Abstract
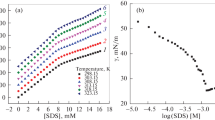
The surface tension of disodium hexadecyl diphenyl ether disulfonate (C16-MADS) was measured at different NaCl concentrations (0.00–0.50 mol L−1) and temperatures (298.0–318.0 K) using the drop-volume method. The results show that, with increasing temperature, the critical micelle concentration (CMC) of C16-MADS increases slightly, but the maximum surface adsorption capacity (Γ max) at the air–water interface decreases. When the concentration of NaCl was increased from 0.00 to 0.50 mol L−1, the CMC of C16-MADS decreased from 1.45 × 10−4 to 4.10 × 10−5 mol L−1, but the surface tension at the CMC (γ cmc) was not affected. When the concentration of NaCl was increased at 298.0 and 303.0 K, the Γ max of C16-MADS increased. When the temperature was increased from 308.0 to 318.0 K, the surface excess concentration (Γ max) of C16-MADS abnormally decreased from 2.26 to 1.41 μmol m−2 with increasing NaCl concentration. The micellization free energy (\(\Delta G_{\text{m}}^{ \circ }\)) decreased from −63.98 to −76.20 kJ mol−1 with increase of temperature and NaCl concentration. The micellar aggregation number (N m) of disodium hexadecyl diphenyl ether disulfonate (C16-MADS) was determined using the molecule fluorescence probe method with pyrene as probe and benzophenone as quencher. The results show that an appropriate N m could be measured only at surfactant concentration above the CMC. The N m increased with an increase in C16-MADS concentration, but the micropolarity in the micelle nucleus decreased. The temperature had little effect on N m. Compared with typical single hydrophilic headgroup surfactants, aggregates of C16-MADS exhibit different properties.




Similar content being viewed by others
References
Rosen MJ, Zhu ZH, Hua XY (1992) Relationship of structure to properties of surfactants. 16. Linear decyldiphenylether sulfonates. JAOCS 69(1):30–33
Liu J (1995) Alkyldiphenyl oxide disulfonates—a new type of high efficiency surfactants. Si Chuan Deterg Cosmet 1:33–38
Bob DT (1991) Reduced adsoption and separation of blended surfactants on sand and clay. J Can Pet Technol 30(2):133–137
Hongzoon KT (1985) Photographic developer composition. US Patent 4565776
Ishii TN, Sasaki K (1987) Alkyldiphenyl ether disufonic acid disodium salts as dispersing agents for wettable pesticides. JP Patent 6299356
Wada TY, Yonemura S (1987) Diphenyl ether sulfonate plant fungicides. JP Patent 62169704
Quencer LB (1996) The detergency properties of mono and disufonated diphenyl oxide surfactants. In: Proceedings of the 4th world surfactant congress. Barcelona, pp 192–201
Loughne TJ, Quencer L (1992) Surfactants for high-performance cleaning. SCCS 68(1):24–30
Doughty DA (1979) Isopiestic compositions of aqueous ionic surfactant systems as a measure of preferential interactions. Application to the determination of micelle aggregation numbers by equilibrium ultracentrifugation. J Phys Chem 83(20):2621–2628
Turro NJ, Yekta A (1978) Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelles. J Am Chem Soc 100(18):5951–5952
Rosen MJ (2004) Surfactants and interfacial phenomena. Wiley, NewYork
Xu HJ, Lv CX, Ye ZW (2005) Preparation and properties of alkyldiphenyl ether disulfonate. Fine Chem 22(1):19–22
Kalyanasundaram K, Thomas JK (1977) Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc 99:2039–2044
Mats A, Shanti S (1983) Size of sodium dodecyl sulfate micelles in the presence of additives. J Colloid lnterface Sci 91(1):256–266
Zhao GX, Zhu BY (2003) Principles of surfactant action. China Light Industry Press, Beijing
Manilal DA, Rosen MJ (1986) Relationship of structure to properties of surfactants. 13. Surface and thermodynamic properties of some oxyethylenated sulfates and sulfonates. J Phys Chem 90:2413–2418
Yan Y, Huang JB, Li ZC (2002) Surface property and aggregation behavior of anionic bolaform surfactant/C12Et3 mixed system. Acta Chim Sin 60(7):1147–1150
Frumkin A (1925) Surface tension curves of the higher fatty acids and the equation of condition of the surface layer. Z Phys Chem 116:466–484
Liu YP (1979) Physical chemistry. China People Education Press, Beijing
Li GZ, Sui H, Zhu WZ (1999) Progress of surfactant study. Chin Surfact Deterg Cosmet 1:24–26
Rosen MJ, Zhu ZH, Gao T (1993) Synergism in binary mixture of surfactants. 11. Mixture containing mono-and disulfonated alkyl-and dialkyldiphenylethers. J Colloid Interface Sci 157(1):254–259
Li YH, Huang JB, Wang CZ (2002) The surface chemical properties of hydrolytic surfactants. In: Proceedings of 2002 international conference on surfactants and detergents. Shen Zhen, pp 195–199
Lana M, Nita M, Pranab KB (1997) Thermodynamics of formation of biological microemulsion (with cinnamic alcohol, Aerosol OT, Tween 20, and water) and kinetics of alkaline fading of crystal violet in them. J Colloid Interface Sci 186(1):1–8
Zhao JX (1999) A new generation of surfactants: geminis. Prog Chem 11(4):348–357
Fang Y, Liu XF, Xia YM (2011) Determination of critical micellar aggregation numbers by steady-state fluorescence probe method. Acta Physicochim Sin 17(9):828–832
Chen JY, Wang GT, Liu JZ (1993) Investigation on the determination of micellar aggregation number by steady-stable fluorescence quenching method. Acta Physicochim Sin 9(4):461–465
Kratohvil JP (1980) Comments on some novel approaches for the determination of micellar aggregation numbers. J Colloid Interface Sci 75(1):271–275
Chen JM, Su TM, Mou CY (1986) Size of sodium dodecyl sulfate micelle in concentrated salt solutions. J Phys Chem 90:2418–2421
Raoul Z, Martin I, Helene L (1997) Alkanediyl-α, ω-bis(dimethylalkylammonium bromide). T. Fluorescence probing studies of micelle micropolarity and microviscosity. Langmuir 13:5552–5557
Huang JB, Zhao GX, Jiang YC (1993) Fluorescence probe study on the mixed catanionic surfactants. Acta Physicochim Sin 9(5):577–580
Acknowledgments
We are grateful for the financial support from the Sinopec Group Jinlin Petrochemical Company and the Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Xu, H., Xu, K. & Wang, D. Surface Chemical Properties and Micellization of Disodium Hexadecyl Diphenyl Ether Disulfonate in Aqueous Solution. J Surfact Deterg 18, 1073–1080 (2015). https://doi.org/10.1007/s11743-015-1741-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1741-3