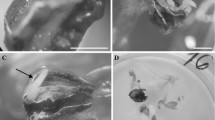
Abstract
This study conducted two experiments involving in vitro anther culture of Zea mays L. The first experiment tested 46 maize genotypes, including inbred lines, single and three-way cross hybrids, and line A188 as control, in three different induction basal media (IMSS, N6 and YPm) for their androgenic responses. The results showed that the embryos were established 2–3 weeks after the anthers of the few responsive genotypes were cultured. Most responsive genotypes produced embryos in at least one of the three basal media; therefore, genotype is more important than the type of medium for androgenesis in maize. The mean number of anthers that developed to embryo ranged from 19 embryos per Petri dish in YPm medium for the cross (DH5 × DH7) genotype to 0 for some maize genotypes. In the second experiment, this research reports for the first time the effect of carbohydrates and polyethylene glycol (PEG) as a non-metabolized osmoticum on the embryogenesis anther culture of maize. The genotype DH5 × DH7 was used for this experiment, and the media were varied by altering sucrose, maltose, and PEG concentrations. Results showed that the maximum embryogenesis (32 embryos per Petri dish) was obtained by YPm basal medium supplemented with 60 gl−1 sucrose + 0.0125 M PEG and 30 gl−1 sucrose + 30 gl−1 maltose + 0.0125 M PEG. The lowest rate of embryogenesis was observed in YPm basal medium with 60 gl−1 maltose and 0.0125 or 0.025 M PEG. Sucrose or a high concentration of maltose was found to be necessary for embryogenesis in anther culture of maize. Therefore, the addition of low levels of PEG and/or different sugars in the experimental design appeared to improve the protocol currently available in the world, especially for anther embryo yield and haploid plant regeneration in maize.



Similar content being viewed by others
References
Abd El-Maksoud MM, Karsai I, Bedo Z (1993) Agronomic traits of wheat lines developed by the doubled haploid, single seed descent and pedigree methods after three cycles of selection. Acta Agron Hung 42:377–382
Achar PNA (2002) Study of factors affecting embryo yields from anther culture of cabbage. Plant Cell Tiss Org Cult 69:183–188
Ahmad T, Abbasi NA, Hafiz IA, Ali A (2007) Comparison of sucrose and sorbitol as main carbon energy source in morphogenesis of peach root stock GF-677. Pak J Bot 39:1264–1275
Al-Ashkar IM (2013) Anther culture response and salt tolerance in some wheat genotypes. Ann Agri Sci 58:139–145
Anonymous, Lab Plant Cell Tissue Cult, 401 Res Group, Inst Genet, Acad Sinica (1975) Primary study on induction of pollen plants of Zea mays. Acta Genet Sin 2:143–145
Antoine-Michard S, Beckert M (1997) Spontaneous versus colchicine-induced chromosome doubling in maize anther culture. Plant Cell Tiss Organ Cult 48:203–207
Aulinger IE (2002) Combination of in vitro androgenesis and biolistic transformation: an approach for breeding transgenic maize (Zea mays L.) lines. Dissertation, Swiss Federal Institute of Technology (ETH), Zurich, Switzerland
Barakat MN, Al-Doss AA, Elshafei AA, Moustafa KA, Ahmed EI (2012) Anther culture response in wheat (Triticum aestivum L.) genotypes with HMW alleles. Cereal Res Commun 40:583–591
Barnabas B, Szakacs E, Karsai I, Bedo Z (2001) In vitro androgenesis of wheat: from fundamentals to practical application. Euphytica 119:211–216
Blanc G, Michaux-Ferriere N, Teisson C, Lardet L, Carron MP (1999) Effects of carbohydrate addition on the induction of somatic embryogenesis in Hevea brasiliensis. Plant Cell Tiss Organ Cult 59:103–112
Blanc G, Lardet L, Martin A, Jacob JL, Carron MP (2002) Differential carbohydrate metabolism conducts morphogenesis in embryogenic callus of Hevea brasiliensis (Mull. Arg.). J Exp Bot 53:1453–1462
Bozhkov PV, Arnold S (1998) Polyethylene glycol promotes maturation but inhibits further development of Piceaabies somatic embryos. Physiol Plant 104:211–224
Büter B (1997) In vitro haploid production in maize. In: Jain SM, Sapory SK, Veilleux RE (eds) In vitro haploid production in higher plants. Kluwer Academic Publishers, Amsterdam, pp 37–71
Calamar A, De Klerk GJ (2002) Effect of sucrose on adventitious root regeneration in apple. Plant Cell Tiss Organ Cult 70:207–212
Chen Y, Dribnenki P (2002) Effect of genotype and medium composition on flax Linum usitatissimum L. anther culture. Plant Cell Rep 21:204–207
Chen Y, Dribnenki P (2004) Effect of medium osmotic potential on callus induction and shoot regeneration in flax anther culture. Plant Cell Rep 23:272–276
Chu CC (1978) The N6 medium and its application to anther culture of cereal crops. In: Proceedings of symposium on plant tissue culture. Science Press, Beijing (Peking), China, pp 43–50
Cowen NM, Johnson CD, Armstrong K, Miller M, Woosley A, Pescitelli S, Skokut M, Belmar S, Petolino JF (1992) Mapping genes conditioning in vitro androgenesis in maize using RFLP analysis. Theor Appl Genet 84:720–724
Dieu P, Beckert M (1986) Further studies of androgenetic embryo production and plant regeneration from in vitro cultured anthers in maize (Zea mays 1.). Maydica 31:245–259
Dodds JH, Roberts LW (1985) Isolation and culture of protoplasts. In: Experiments in plant tissue culture. Cambridge University Press, New York, pp 156–171
Dolezel J (1997) Application of flow cytometry for the study of plants genomes. J Appl Genet 38:285–302
Dong HD, Zhong JJ (2001) Significant improvement of taxane production in suspension cultures of Taxus chinensis by combining elicitation with sucrose feed. Biochem Eng J 8:145–150
Fu X, Yang S, Bao M (2008) Factors affecting somatic embryogenesis in anther cultures of Chinese pink (Dianthus chinensis L.). In Vitro Cell Dev Biol Plant 44:194–202
Genovesi AD, Collins GB (1982) In vitro production of haploid plants of corn via anther culture. Crop Sci 22:1137–1144
Herbers K, Meuwly P, Métraux JP, Sonnewald U (1996) Salicylic acid independent induction of pathogenesis-related protein transcripts by sugars is dependent on leaf developmental stage. FEBS Lett 397:239–244
Hoekstra S, Van Bergen S, Van Brouwershaven IR, Schilperoort RA, Heidekamp F (1996) The interaction of 2,4-D application and mannitol pre-treatment in anther and microspore culture of Hordeu mvulgare L. cv. Igri. J Plant Physiol 148:696–700
Ilic-Grubor K, Attree SM, Fowke LC (1998) Induction of microspore-derived embryos of Brassica napus L. with poly-ethylene glycol (PEG) as osmoticum in a low sucrose medium. Plant Cell Rep 17:329–333
Iraki NM, Bressan RA, Hasegawa PM, Carpita NC (1989) Alteration of the physical and chemical structure of the primary cell wall of growth-limited plant cells adapted to osmotic stress. Plant Physiol 91:39–47
Jain N, Babbar S (2003) Effect of carbon source on the shoot proliferation potential of epicotyl explants of Syzygium cuminii. Biol Plant 47:133–136
Jumpatong C, Boonyai P, Sangduen N, Thiraporn R, Saisingtong S, Buter B (1996) Anther culture, a new tool for generation of doubled haploid, homozygous maize in Thailand. Thai J Agri Sci 29:469–487
Kao KN (1993) Viability, cell division and microcallus formation of barley microspores in culture. Plant Cell Rep 12:366–369
Karsai I, Bedo Z (1998) Relationship between anther culture response and aluminium tolerance in wheat (Triticum aestivom L.). Euphytica 100:249–252
Koch K (1996) Carbohydrate-modulated gene expression in plants. Ann Rev Plant Physiol 47:509–540
Kovacs G, Barnabas (1997) Selection for frost tolerance in anther culture-derived embryos and regeneration of frost-tolerant fertile DH plants in winter wheat. Acta Agron Hung 45:285–293
Ku MK, Cheng WC, Juo LC, Kuan YL, An HP, Huang CH (1978) Induction factors and morpho-cytological characteristics of pollen derived plants in maize (Zea mays L). In: Proceedings of symposium on plant tissue culture. Science Press, Beijing (Peking), China, pp 35–42
Last DI, Brettell RIS (1990) Embryo yield in wheat anther culture is influenced by the choice of sugar in the culture medium. Plant Cell Rep 9:14–16
Luckett DJ, Dantey NL (1992) Utilization of microspore culture in wheat and barley improvement. Aust J Bot 40:807–828
Miao SH, Kuo CS, Kwei YL, Sun AT, Ku SY, Lu WL, Wan YY, Chen ML, Wu MK, Hang L (1978) Induction of pollen plants of maize and observations on their progeny. In: Proceedings of symposium on plant tissue culture. Science Press, Beijing (Peking), China, pp 23–33
Mohammadi PP, Moieni A, Javaran MJ (2007) Colchicine induced embryogenesis and doubled haploid production in maize (Zea mays L.) anther culture. Iran J Biotech 5:140–146
Moing A, Carbonne F, Rashad MH, Gaudillere JP (1992) Carbon fluxes in mature peach leaves. Plant Physiol 100:1878–1884
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nitsch C, Andersen S, Godard M, Neuffer MG, Sheridan WF (1982) Production of haploid plants of Zea mays and Pennisetum through androgenesis. In: Earle KD, Demarly Y (eds) Variability in plants regenerated from tissue culture. Praeger, New York, pp 69–91
Obert B, Preťová A, Büter B, Schmid JE (2000) Effect of different saccharides on viability of isolated microspores and androgenic induction in Zea mays. Biol Plant 43:125–128
Park SG, Ubaidillah M, Kim KM (2013) Effect of maltose concentration on plant regeneration of anther culture with different genotypes in rice (Oryza sativa L.). Am J Plant Sci 4:2265–2270
Petolino JF, Jones AM (1986) Anther culture of elite genotypes of maize. Crop Sci 26:1072–1074
Roberts-Oehlschlager SL, Dunwell JM, Faulks R (1990) Changes in the sugar content of barley anthers during culture on different carbohydrates. Plant Cell Tiss Organ Cult 22:77–85
Saisingtong S, Schmid JE, Stamp P, Buter B (1996) Colchicine-mediated chromosome doubling during anther culture of maize (Zea mays L.). Theor Appl Genet 92:1017–1023
Sharma PC, Gupta PK (1982) Karyotypes in some pulse crops. Nucleus 25:181–185
SteinerI N, Vieira FN, Maldonado S, Guerra MP (2005) Effect of carbon source on morphology and histodifferentiation of Araucaria angustifolia embryogenic cultures. Braz Arch Biol Technol 48:895–903
Swedlund B, Locy RD (1993) Sorbitol as the primary carbon source for the growth of embryogenic callus of maize. Plant Physiol 103:1339–1346
Teixerira Da Silva JA (2004) The effect of carbon source on in vitro organogenesis of chrysanthemum thin cell layers. Bragantia 63:165–177
Thomson M, Thorpe TA (1987) Metabolic and non-metabolic roles of carbohydrates. In: Bong JM, Durzan DJ (eds) Cell tissue culture forestry. Martinus Nijhoff Publisher, Dordrecht, pp 89–112
Thorn EC (1988) Effect of melibiose and polyethylene glycol on anther culture response of Barley. In: International congress of genetic manipulation in plant breeding: biotechnology for the breeder. Helsingoer (Elsinore), Denmark, pp 80
Thorpe TA (1980) Organogenesis in vitro: structural, physiological and biochemical aspects. In: Vasil KI (ed) International review of cytology: perspectives in plant cell and tissue culture. Acedemic Press, New York, pp 71–111
Ting YC, Yu M, Zheng WZ (1981) Improved anther culture of maize (Zea mays). Plant Sci Lett 23:139–145
Vitova L, Stodulkova E, Bartonickova A, Lipavska H (2002) Mannitol utilization by celery (Apium graveolens) plants grown under different conditions in vitro. Plant Sci 163:907–916
Wan Y, Widholm JM (1992) Formation of multiple embryo-like structures from single microspores during maize anther culture. Plant Cell Rep 11:529–531
Wojnarowiez G, Caredda S, Devaux P, Sangwan R, Clément C (2004) Barley anther culture: assessment of carbohydrate effects on embryo yield, green plant production and differential plastid development in relation with albinism. J Plant Physiol 161:747–755
Yadollahi A, Abdollahi MR, Moieni A, Danaee M (2011) Effects of carbon source, polyethylene glycol and abscisic acid on secondary embryo induction and maturation in rapeseed (Brassica napus L.) microspore-derived embryos. Acta Physiol Plant 33:1905–1912
Yaseen M, Ahmad T, Sablok G, Standardi A, Ahmad Hafiz I (2013) Role of carbon sources for in vitro plant growth and development. Mol Bio Rep 40:2837–2849
Acknowledgments
The authors are grateful for the financial support of the Lorestan University Research council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J van Huylenbroeck.
Rights and permissions
About this article
Cite this article
Ismaili, A., Mohammadi, P.P. Effect of genotype, induction medium, carbohydrate source, and polyethylene glycol on embryogenesis in maize (Zea mays L.) anther culture. Acta Physiol Plant 38, 74 (2016). https://doi.org/10.1007/s11738-016-2085-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2085-y