Abstract
Conservation of important plant germplasm is often difficult due to the specific growth requirements of genetically diverse species including in vitro culture collections. Recently the mesos components (CaCl2, MgSO4, KH2PO4) of Murashige and Skoog medium were identified as one of the most influential groups of nutrients for five pear genotypes. To determine if this requirement also applied to a larger germplasm collection, 18 genotypes in six species were screened. Shoot quality, shoot length, leaf spots and leaf color were the most affected responses. Seven of nine Pyrus communis cultivars had improved shoot quality, five had significantly longer shoots, better leaf color and fewer leaf spots while two had more shoots. Two of the four Pyrus pyrifolia cultivars had improved shoot quality while three had better leaf color and fewer leaf spots. Pyrus calleryana ‘Capital’, Pyrus cordata and Pyrus ussuriensis ‘Harbin’ had longer shoots while Pyrus koehnei had less callus. P. ussuriensis ‘Hang Pa Li’ was the only genotype where shoot quality declined at high mesos concentrations. Quantitative ion analysis detected substantially higher concentrations of Ca, Mg and K, but significantly less Fe, in the shoots cultured on increased mesos compared to controls. This study confirms that increased mesos improved growth of P. communis and P. pyrifolia cultivars, but produced fewer significantly improved responses for four other species.





Similar content being viewed by others
Abbreviations
- Mesos:
-
Components CaCl2·2H2O, MgSO4·7H2O, KH2PO4 (monobasic)
- MS:
-
Murashige and Skoog Medium
References
Bell RL, Reed BM (2002) In vitro tissue culture of pear: advances in techniques for micropropagation and germplasm preservation. Acta Hortic 596:412–418
Bell RL, Srinivasan C, Lomberk D (2009) Effect of nutrient media on axillary shoot proliferation and preconditioning for adventitious shoot regeneration of pears. In Vitro Cell Dev Biol Plant 45:708–714
Bondarev N, Reshetnyak O, Nosov A (2003) Effects of nutrient medium composition on development of Stevia rebaudiana shoots cultivated in the roller bioreactor and their production of steviol glycosides. Plant Sci 165:845–850
Bosela MJ, Michler CH (2008) Media effects on black walnut (Juglans nigra L.) shoot culture growth in vitro: evaluation of multiple nutrient formulations and cytokinin types. In Vitro Cell Dev Biol Plant 44:316–329
Bucher M et al (2007) Molecular physiology of the mineral nutrition of the potato. Potato Biology and Biotechnology. Elsevier Science B.V, Amsterdam, pp 311–329
Design-Expert (2010) Stat-Ease, Inc., Minneapolis
Driver JA, Kuniyuki AH (1984) In vitro propagation of Paradox walnut rootstock. HortScience 19:507–509
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Lloyd G, McCown B (1980) Commercially feasible micropropagation of mountain laurel Kalmia latifolia, by use of shoot-tip culture. Comb Proc Int Plant Prop Soc 30:421–427
Marschner H (1995) Mineral nutrition of higher plants 2edn. Academic Press, New York
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures Physiol Plant 15:473–497
Niedz RP, Evens TJ (2007) Regulating plant tissue growth by mineral nutrition. In Vitro Cell Dev Biol Plant 43:370–381
Niedz RP, Hyndman SE, Evens TJ (2007) Using a Gestalt to measure the quality of in vitro responses. Sci Hortic 112:349–359
Preece J (1995) Can nutrient salts partially substitute for plant growth regulators? Plant Tiss Cult Biotech 1:26–37
Quoirin M, Lepoivre P (1977) Improved media for in vitro culture of Prunus. Acta Hortic 78:437–442
Reed BM (1999) The in vitro genebank of temperate fruit and nut crops at the National Clonal Germplasm Repository-Corvallis. In: Engelmann F (ed) Management of Field and In Vitro Germplasm Collections. International Plant Genetic Resources Institute, Rome, pp 132–135
Reed BM, Paynter CL, DeNoma J, Chang Y (1998) Techniques for medium-and long-term storage of Pyrus L. genetic resources. Plant Gen Resour Newsl 115:1–4
Reed BM, DeNoma JS, Wada S, Postman JD (2013a) Micropropagation of pear (Pyrus sp). In: Lambardi M, Ozudogru EA, Jain SM (eds) Protocols for micropropagation of selected economically important horticultural plants. Humana Press-Springer, NY, p 554
Reed BM, Wada S, DeNoma J, Niedz RP (2013b) Improving in vitro mineral nutrition for diverse pear germplasm. In Vitro Cell Dev Biol Plant 49:343–355. doi:10.1007/s11627-013-9504-1
Reed BM, Wada S, DeNoma J, Niedz RP (2013c) Mineral nutrition influences physiological responses of pear in vitro. In Vitro Cell Dev Biol Plant 49:699–709
Sallanon H, Isaka H, Dimon B, Ravel C, Chagvardieff P (1997) CO2 exchanges and nutrient uptake during multiplication and rooting of micropropagated Juglans regia plantlets. Plant Sci 124:107–116
Singha S (1986) Propagation of fruit trees using tissue culture. Pomona 19:4–5
Skirvin RM (1981) The tissue culture of fruit crops. In: Conger BV (ed) Cloning agricultural plants via in vitro techniques. So. Orchard, Urbana, pp 51–139
Troyanos YE, Hipps NA, Moorby J, Kingswell G (2000) The effects of external potassium and magnesium concentrations on the magnesium and potassium inflow rates and growth of micropropagated cherry rootstocks, ‘F.12/1’ (Prunus avium L.) and ‘Colt’ (Prunus avium L.) x Prunus pseudocerasus L.). Plant Soil 225:73–82
Wada S, Niedz RP, DeNoma J, Reed BM (2013) Mesos components (CaCl2, MgSO4, KH2PO4) are critical for improving pear micropropagation. In Vitro Cell Dev Biol Plant 49:356–365
Wada S, Niedz RP, Reed BM (2014) Determining nitrate and ammonium requirements for optimal in vitro response of diverse pear species. In Vitro Cell Dev Biol Plant (in press). doi:10.1007/s11627-015-9662-4
Zhao H, Gu N (1990) Pear. In: Chen Z, Evans DA, Sharp WR, Ammirato PV, Sondahl MR (eds) Handbook of Plant Cell Culture, vol 6. McGraw-Hill, New York, pp 264–277
Acknowledgments
We thank NCGR lab personnel for assistance with collection of the data. This project was funded by a grant from the Oregon Association of Nurseries and the Oregon Department of Agriculture and by USDA-ARS CRIS project 5358-21000-0-38-00D.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. K. Nagar.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11738_2014_1754_MOESM1_ESM.docx
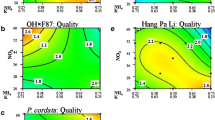
ESM 1. Trend lines of the interaction graph for additional responses of nine P. communis genotypes. Ratings: shoot multiplication (shoots counted), leaf color (1 green 2 yellow 3 red or brown), leaf spotting/necrosis (rated 1 absent, 2 minor, 3 major), callus (1 absent, 2 ≤ 3 mm, 3 > 3 mm) and leaf size (1 small, 2 medium, 3 large).ESM 2. Trend lines of the interaction graph for additional responses of four P. pyrifolia genotypes. Ratings: shoot multiplication (shoots counted), leaf color (1 green 2 yellow 3 red or brown), leaf spotting/necrosis (rated 1 absent, 2 minor, 3 major), callus (1 absent, 2 ≤ 3 mm, 3 > 3 mm) and leaf size (1 small, 2 medium, 3 large).ESM 3. Trend lines of the interaction graph for additional responses of P. koehnei, P. calleryana ‘Capital’, P. cordata, P. ussuriensis ‘Hang Pa Li’ and ‘Harbin’. Ratings: shoot multiplication (shoots counted), leaf color (1 green 2 yellow 3 red or brown), leaf spotting/necrosis (rated 1 absent, 2 minor, 3 major), callus (1 absent, 2 ≤3 mm, 3 > 3 mm) and leaf size (1 small, 2 medium, 3 large). (DOCX 1104 kb)
Rights and permissions
About this article
Cite this article
Wada, S., Maki, S., Niedz, R.P. et al. Screening genetically diverse pear species for in vitro CaCl2, MgSO4 and KH2PO4 requirements. Acta Physiol Plant 37, 63 (2015). https://doi.org/10.1007/s11738-014-1754-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-014-1754-y