Abstract
According to regular reports, one of the most serious diseases of winter cereal and grass varieties in moderate and cold climatic areas is pink snow mould caused by Microdochium nivale. Currently, the resistance of the economically important cereal species as triticale is not satisfactory. Moreover, there is no efficient strategy of protection against this pathogen and the understanding of plant resistance mechanisms is rather poor. Presented paper for the first time shows the cytological analysis of M. nivale infection in model triticale varieties by the use of fluorescent and light microscopy in combination with fluorescent dyes and hydrogen peroxide staining. Both, the infection level and the dynamic of the process varied for tested genotypes confirming the field and laboratory data of their different resistance to this pathogen. Moreover, our analysis showed that in both cultivars cold-hardening of seedlings delayed the mycelium growth. In both cultivars, hyphal walls and fungal penetration sites were visualized in crowns, leaf sheaths and leaves of hardened and non-hardened inoculated seedlings. For the first time the presence of the haustoria produced by M. nivale was confirmed in those tissues. Single infection hyphae usually penetrated into the host tissues via stomatal apparatuses were accompanied by the efflux of hydrogen peroxide. The data show a great potential of fluorescence techniques in studying the host plant–pathogen interactions providing a better insight into plant defence reactions that may allow elaboration of the efficient breeding strategies aimed at increasing resistance to this pathogenic fungus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to regular reports, one of the most serious diseases of winter cereal varieties and perennial grasses in moderate and cold climatic areas is pink snow mould caused by Microdochium nivale (Fr.) Samuels & Hallett (Tronsmo et al. 2001; Pronczuk et al. 2003). The main hosts of M. nivale are wheat, rye, barley, oats, turf and forage grasses (Tronsmo et al. 2001). Among others, M. nivale infects winter triticale (Cichy and Maćkowiak 1993; Golebiowska and Wedzony 2009), the man-made cereal obtained by initial crossing wheat (Triticum vulgare) with rye (Secale cereale) and further stabilization of the allopolyploid with the use of laboratory methods (Ryöppy 1997). The economic importance of triticale grows fast but both the quality and the quantity of its yield is regularly under the threat of pathogenic infection.
Among the most important cereal cultivars the resistance to M. nivale is not satisfactory. Moreover, available fungicides are not sufficiently efficient and have negative impact on the environment. Published data (Nakajima and Abe 1996; Ergon et al. 1998; Golebiowska and Wedzony 2009) report that maximal snow mould resistance develops only in cold-hardened plants and varies among genotypes. Whereas the majority of pathogens are less aggressive and unable to infect plants at freezing temperatures, some psychrophilic pathogens, including M. nivale, are able to invade plants under these conditions and cause significant damage (Tronsmo et al. 2001). The infection is even easier as plants are more susceptible to pathogen infection when even a slight freezing injury has occurred.
On the other hand, exposure to cold may increase tolerance to many other stresses. The mechanism of this phenomenon is not entirely understood, usually explained by unspecificity of defence reactions induced under the stress treatment (Antikainen et al. 1996; Hincha et al. 1997). Among them, rapid generation of reactive oxygen species (ROS) is the very early response to almost each kind of stress factors. ROS, as highly active molecules, can be very dangerous and they can cause damages to almost every organic constituent of the living cell. Nevertheless, some of them, especially hydrogen peroxide, can act as a signaling molecule involved in many processes associated with plant growth and development (Pitzschke and Hirt 2006). Recently, H2O2 has been postulated to play a central and manifold role in plant resistance response to fungal pathogens (Kuzniak and Urbanek 2000).
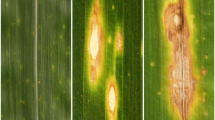
The first symptoms of M. nivale infection are normally water soaked areas on leaves, followed by a gradual chlorosis of plants in darkness. Under the strong light dark spots can occur. Other typical symptoms include brown lesions on leaves (Browne et al. 2006). At higher temperatures, M. nivale can also cause stem rot and head blight or fusarium ear blight of cereals (Tronsmo et al. 2001).
Recently, much effort has been directed towards elucidating the cytological events underlying the process of plants colonization by different fungi. The papers mainly illustrate infection initiated by fungal spores and the literature describing the cytological analysis of the infection in cereals is fragmentary (Clement and Parry 1998; Kumar et al. 2002; Oren et al. 2003; Jackowiak et al. 2005; Kang et al. 2007). There is no cytological data describing the infection caused by mycelial inoculum of M. nivale, including the early phases of pathogenesis. Thus, the aim of the present study was to analyse the interaction between M. nivale hyphae and the host cells of resistant and susceptible plants in controlled conditions by the use of light and fluorescence microscopy. Model genotypes were selected in our previous experiments (Golebiowska and Wedzony 2009). Such studies, providing better insight into mechanisms of the host–pathogen interactions would be highly expected and may allow elaborating efficient breeding strategies towards increased pink snow mould resistance.
Materials and methods
Plant material
The experiments were carried out according to the ‘cold chamber’ method described by Cormack and Lebeau (1959) modified by Pronczuk et al. (2003) and Golebiowska and Wedzony (2009). Two cultivars of hexaploid winter triticale (×Triticosecale Wittm., 2n = 6x = 42) that differed in resistance to M. nivale in previous experiments, both at laboratory and field conditions (Golebiowska and Wedzony 2009), were used for the present study, i.e. susceptible cv. ‘Magnat’ (Danko Hodowla Roslin Ltd.) and partly resistant cv. ‘Hewo’ (Hodowla Roslin Strzelce Ltd.). Seeds were surface-sterilized using 96% ethanol (3 min), 0.05% mercury (II) chloride (1.5 min) and 25% solution of commercial sodium hypochlorite (Domestos, 15 min). After washing in sterile water, seeds were germinated on moisturized sterile filter paper for 2 days at 26°C in darkness. Healthy seedlings were transferred to 20 × 20 cm pots (16 plants per pot) filled with the sterile mixture of soil, peat and sand (2:2:1, v:v:v) and grown in climatic chamber at temperature 16/12°C, 8/16 h (day/night) with the light intensity 100 ± 10 μmol [quantum] m−2 s−1 PAR, and relative humidity 60–67% for 7 days.
As previously described (Golebiowska and Wedzony 2009), 7-day-old plants were subjected first to 14-day prehardening period (12/12°C, 8/16 h day/night) and then to 28-day hardening (4/4°C, 8/16 h day/night). Control, non-hardened plants were grown at 16/12°C, 8/16 h (day/night) for 21 days until acquiring the stage of development morphologically similar to cold-hardened plants before inoculation. Both prehardening and hardening treatments were curried out at the light intensity 100 ± 10 μmol [quantum] m−2 s−1 PAR, and relative humidity 60–67%.
Inoculum
Inoculum was produced according to the method described by Pronczuk et al. (2003). Mycelium of the monosporal isolate of M. nivale No. 38z/5a/01 (Dr Pronczuk, Institute of Plant Breeding and Acclimatization, Radzikow, Poland) highly aggressive towards cereals was grown on 1 cm thick layer of the Potato Dextrose Agar (PDA) medium (Sigma-Aldrich, Cat. No. P2182) in sterile 9 cm of diameter Petri dishes at 21°C in darkness for 10 days. Fourth part of the mycelium from each PDA dish was transferred to a flask with watered sterile mixture of soil, peat and sand (2:2:1; v:v:v), containing 5% w/w of ground wheat seeds. The mycelium was then cultured for 14 days at 21°C in darkness. For further experiments the isolate was stored as mycelium on PDA pieces at −80°C. According to Browne et al. (2006) the vitality of the stored isolate was checked before each use by measuring its growth rate on PDA medium at 21°C.
Plant inoculation
Soil-borne mycelium was gently mixed and displayed on soil surrounding plants (1 g per one plant). Inoculation was performed on hardened and non-hardened (the control of the effect of hardening) seedlings of both cultivars. Three pots for each cultivar-pretreatment combination were inoculated and three pots remained not inoculated as the control of cold-chamber conditions. Each pot was covered by moist paper and black plastic bags to imitate snow cover and to keep high humidity and darkness. The plants were incubated for 7 days at 4°C, in darkness in the cold chamber according to Pronczuk et al. (2003) and Golebiowska and Wedzony (2009).
Sampling and microscopy
Inoculated and non-inoculated plants were uprooted from each pot and washed gently under the tap water 1, 3, 6, 9 h after wet covers were applied on the same inoculation day. The samples were also collected 1, 3, 5 and 7 days after inoculation. Stained fragments and cross-sections of living seedlings (leaves, leaf sheaths, crowns, and roots) were analysed on slides.
For the visualization of M. nivale hyphae, two fluorescent dyes: Calcofluor White (O’Brien and McCully 1981) and Aniline Blue (Mlodzianowski and Wozny 1990) were used. CalcofluorWhite stain (18909, stock solution at 1% w/v in H2O; BioChemika) was used at a final concentration of 0.01% for 5 min. Aniline Blue (415049; stock solution at 0.5% w/v in H2O; Sigma-Aldrich) was used at a final concentration of 0.05%, pH 8.2 for 5 min. M. nivale hyphae were analysed under UV fluorescence (excitation filter 365 nm, dichroic mirror 395 nm, barrier filter 420 nm).
To visualize H2O2, 0.1% DAB (3,3′-diaminobenzidine tetrahydrochloride; Sigma-Aldrich, St. Louis, MO, Cat. No. D12384) (w/v, pH 3.8) staining was carried out as previously described by Thordal-Christensen et al. (1997). Leaves of non-hardened and cold-hardened plants sampled on the 7th day post-inoculation were soaked in the DAB solution and placed in a desiccator (0.8 MPa) for 10 min. Then the DAB solution was replaced with a fresh one and the whole procedure was repeated. After that, incubated leaves were allowed to take up the DAB solution for 1 h on the bench top away from strong lighting. Next, leaves were placed in 80% ethanol (60°C, for 8 h).
Cytological analyses were performed with the microscope Nikon Eclipse E-600 equipped with DIC system and high sensitivity digital camera DXM 1200F. Images were acquired and processed using software programs including NIS-Elements (AR 2.10, Laboratory Imaging system, Ltd.), PHOTO-PAINT 9 and Power Point (2003).
Results
Microscopic observations
Microscopic analysis of M. nivale development in different organs of relatively resistant and susceptible triticale seedlings (cv. ‘Hewo’ and cv. ‘Magnat’, respectively) clearly showed the infection dynamic of M. nivale. Moreover, our analysis showed that in both tested cultivars hardening of seedlings delayed M. nivale mycelium growth (see description below). Fungus growth in hardened and non-hardened seedlings was observed in: (I) endoderm and cortex cells of crowns, (II) epidermal cells of leaves, and (III) leaf sheaths. The fungus grew upward from crowns at the soil level and then infected outer sheaths and leaves of seedlings. The spread of the pathogen proceeded by the cell wall penetration followed by the development of hyphae forming haustoria within the invaded, living epidermal host cell in leaves or endoderm and cortex cells of crowns. In many cases, the pathogen penetration via stomata in leaves (Figs. 1, 2, 3) was observed. Microscopic observation of tissues of non-inoculated seedlings did not reveal any serious tissue damage nor phenolic compounds accumulation.
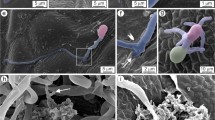
Cytological analysis of the triticale (×Triticosecale) host response after inoculation with Microdochium nivale in non-hardened (a–h) and hardened (i–m) seedlings of cv. ‘Magnat’. Non-hardened seedlings 1 h (a), 3 h (b), 1 day (c), 3 days (d, e), 5 days (f), and 7 days (g, h) after inoculation. a Hyphae in cortex cells of stem base (arrowheads indicate intracellular hyphae producing haustoria). Stem base cross-section. b Single hyphae on the leaf surface. Red-autofluorescence of chlorophyll. c Autofluorescence of phenolic compounds (yellow) accumulated in epidermal cells of leaf. Blue-autofluorescence of cellulose, red-autofluorescence of chlorophyll. d Early phase of stomata apparatus penetration by hyphae. e Autofluorescence of phenolic compounds (light yellow-blue) accumulated in leaf epidermal cells and in cells next to the stomata apparatus. Blue-autofluorescence of cellulose. f Hyphae on the surface of cortex cells of crowns and on leaf sheaths. g Dense network of hyphae on the surface of leaf epidermis cells (yellow-white). Red-autofluorescence of chlorophyll. h Dense network of hyphae on the surface of the youngest leaf (blue). Hardened seedlings 5 days (i, j), and 7 days (k–m) after inoculation. i Dense network of hyphae on the surface of leaf epidermis cells. Red-autofluorescence of chlorophyll. j Hyphae in cortex cells of crown. Crown-cross section. k Dense network of hyphae in leaf epidermis cells (yellow). Red-autofluorescence of chlorophyll. l Single hyphae in the youngest leaf (blue, arrowhead). m Intercellular hyphae in cortex cells of crown (arrowheads). Bars 10 μm (c–l), 20 μm (b, m). Nomarski optics (a); Fluorescence microscopy in UV light (b–m) (colour figure online)
Cytological analysis of the triticale (×Triticosecale) host response after inoculation with Microdochium nivale in non-hardened (a–e) and hardened (f–k) seedlings of cv. ‘Hewo’. Non-hardened seedlings 3 days (a–b), 5 days (c) and 7 days (d–e) after inoculation. a Hyphae producing haustoria in leaf epidermis cells (blue). b Haustorium next to the stomata apparatus. c Dense network of hyphae in leaf epidermis cells (yellow-white). Red-autofluorescence of chlorophyll. d Crown penetrated by hyphae (arrowheads). e Hyphae next to the root and root hairs (arrowhead). Hardened seedlings 3 days (f–h), 5 days (j–k) after inoculation. f Dense network of hyphae in endodermis and cortex cells of crown. g Haustoria. h Intracellular hyphae. i Hyphae on the surface of the youngest leaf (blue). j Dense network of hyphae on the surface of leaf epidermis cells (blue). Red-autofluorescence of chlorophyll. k Early phase of stomata apparatus penetration by hyphae (blue). Red-autofluorescence of chlorophyll. Bars 10 μm (e, i, j), 20 μm (f, g, k), 40 μm (h), 60 μm (b), 100 μm (a, d). Nomarski optics (d, h); Fluorescence microscopy in UV light (a–c, e, k) (colour figure online)
Hydrogen peroxide detection in cold-hardened seedlings of ‘Magnat’. a–c Seedlings inoculated with Microdochium nivale and stained with DAB. a Stomata stained with DAB. b M. nivale hyphae penetrating the stomata apparatus. c M. nivale hyphae penetrating leaf mesophyl cells of hardened ‘Magnat’. d–e Non-inoculated seedlings stained with DAB. d The surface of the leaf. e Stomata apparatus. The brown colour around the hyphae penetration sites indicates the places of H2O2 generation. Bars 10 μm (a, b), 60 μm (c, e), 100 μm (d) (colour figure online)
Cytological analyses of inoculated plants showed that non-hardened ‘Magnat’ plants were much more susceptible to M. nivale infection than cold-hardened ones at the same developmental stage. In crowns of non-hardened ‘Magnat’ plants, fungal hyphae were found as early as 1 h after inoculation. These hyphae penetrated cortex cells and produced haustoria (Fig. 1a). Two hours later, single hyphae were visible on leaves (Fig. 1b). At the same time, in hardened plants of ‘Magnat’, cortex cell of crown was still not infected; however, single fungal hyphae were detected on leaves surface. One day after inoculation, on the leaves surface of both hardened and non-hardened control ‘Magnat’ plants, a dense network of hyphae was observed. In non-hardened plants, fungal expansion was accompanied with accumulation of phenolic compounds, detected in epidermal leaf cells, close to the stomata apparatus (Fig. 1c). On the 3rd day after inoculation of non-hardened ‘Magnat’, hyphae started penetration of stomata (Fig. 1d). At the same time, older leaves of hardened ‘Magnat’ did not shown any signs of infection except the accumulation of phenolic compounds located close to the stomata in leaf epidermal cells (Fig. 1e). The youngest leaves of hardened ‘Magnat’ seedlings were the first infection sites, from which the fungus started to widespread. On the surface of such leaves, close to the crowns, single hyphae were visible. Starting from the 5th day after inoculation, serious infection symptoms (numerous hyphae on the surface or in cortex cells of crowns, epidermal cells of leaves and in leaf sheaths) in non-hardened as well as hardened ‘Magnat’ seedlings were observed (Fig. 1f–m).
Microscopic observation of both non-hardened and hardened seedlings of ‘Hewo’ did not reveal any signs of pathogen infection during the first 24 h after inoculation with M. nivale (Fig. 2). On the 1st day after inoculation, single hyphae on leaves of non-hardened plants were detected. Two days later, infection developed both in non-hardened and hardened seedlings, and multiple hyphae were observed in endodermis and cortex cells of the stem base as well as on the surface of leaves (Fig. 2a, b, f–h). In non-hardened ‘Hewo’ seedlings haustoria producing hyphae were observed in epidermis cells of leaves. Those hyphae grew through the stomata into the lower leaf cell layers (Fig. 2a, b). From the 5th day after inoculation, branches of intercellular and intracellular hyphae protruded from the outer epidermal cells of leaves, sheaths and cortex cells of the stem base in both non-hardened and hardened ‘Hewo’ seedlings (Fig. 2c, i–k). Later, on the 7th day after inoculation in non-hardened ‘Hewo’, fungal hyphae had formed haustoria, projected into dipper layers of host crown cells (Fig. 2d). At the same time, multiple hyphae surrounded roots and root hairs (Fig. 2e). As the effect of the pathogen infection, cells often were plasmolysed and the contact sites were darken and soaked.
Detection of H2O2
Not inoculated seedlings of both cultivars did not accumulate hydrogen peroxide at the experimental conditions. On the 7th day after infection, DAB visualization of hydrogen peroxide revealed its accumulation in both genotypes on 7th day of infection at M. nivale hyphae penetration sites in epidermal and mesophyl cells (Fig. 3a–c). In some cases, generation of H2O2 was also observed in close vicinity to the infection sites. DAB analyses of the initial stages of infection confirmed that the penetration into leaf tissues occurred via stomata apparatuses as was observed by the fluorescence microscopy techniques.
Discussion
The majority of published data on the initiation of fungal infection are based on studies of germination of fungal spores on plant organs. Such data were published, for instance, in the case of the maize infection caused by Fusarium verticillioides (Oren et al. 2003) or Bipolaris sorokiniana (Kumar et al. 2002), infection of wheat caused by Fusarium culmorum, F. graminearum and M. nivale (Clement and Parry 1998; Jackowiak et al. 2005; Kang et al. 2007). However, the infection development initiated from spores differs from the process induced by M. nivale mycelium, which is the main source of infection in winter conditions (Tronsmo et al. 2001).
There is no cytological data describing the infection of cereal seedlings caused by mycelial inoculum of M. nivale. With the refinement of cytological techniques and the application of these approaches to pathosystems under the study, several questions regarding the relationship between the pathogen and its target host have been addressed.
Cytology of infection
The present paper confirms previous ‘cold chamber’ experiments with the same triticale genotypes (Golebiowska and Wedzony 2009): winter triticale cultivars ‘Hewo’ and ‘Magnat’ differed in their susceptibility to M. nivale infection. Moreover, cytological observations revealed that non-hardened ‘Hewo’ was susceptible to pathogenic fungus infection 1 day later than non-hardened ‘Magnat’. Similarly, hardened ‘Hewo’ was more resistant to M. nivale then hardened ‘Magnat’. Based on our observation, we can conclude that plant resistance to M. nivale attack depends on the ability of the genotype to acquire cold-induced resistance. This is in agreement with results obtained by Ergon et al. (1998) on winter wheat, by Pronczuk et al. (2003) in winter rye and our earlier results (Golebiowska and Wedzony 2009). According to our results it can be also concluded that some components of partial pink snow mould resistance in winter triticale may be expressed prior to cold hardening in relatively resistant genotype. Similar observation was documented by other authors for winter wheat (Ergon and Tronsmo 2006).
Based on received results it can be supposed that hardening strengthens resistance mechanism even in ‘Magnat’ triticale seedlings, described by Golebiowska and Wedzony (2009) and Golebiowska et al. (2010) as susceptible to M. nivale. Our work demonstrated that hardening visibly inhibited penetration of fungal hyphae into leaf and crown tissue. The positive effect of cold-hardening on resistance to M. nivale infection was mainly detectable in ‘Hewo’, described by Golebiowska and Wedzony (2009); Golebiowska et al. (2010) as partly resistant to pink snow mould. Our data are in agreement with findings of other authors, and they show cold-hardening as the trigger of cereal resistance to snow mould due to an enhanced expression of defense mechanisms in these plants (in Tronsmo et al. 1993; Hiilovaara-Teijo et al. 1999; Gaudet et al. 2000; Browne et al. 2006; Golebiowska and Wedzony 2009). The results of cytological analyses lend support to the above-mentioned findings.
Our studies indicate that M. nivale mycelium can infect leaves, leaf sheaths and crowns in non-hardened and cold-hardened plants and that the infection dynamics vary between model cultivars and between temperature treatments. In the presented work, crowns and the youngest leaves of both hardened and non-hardened plants were the first sites, where hyphae of M. nivale were detected in both triticale cultivars. Two hours later, the fungal hyphae were observed on leaves surfaces. The penetration of the leaf occurred through the stomata apparatus. Hyphae entered directly through the stomata in epidermis of leaves and often formed haustoria projected into stomata cells. Our results correspond to studies reported by Gaudet and Kokko (1985) on cottony snow mould caused by the low-temperature basidiomycetes (LTB) which also penetrates into leaves through the stomata. Similarly, anatomy studies on rye seedlings revealed leaves as the main places of M. nivale infection (Koczowska and Packa 1986).
On the basis of our cytological analysis of inoculated plants it could be concluded that the infection can spread from crowns to leaves sheaths and later to the leaves. The similar conclusions were drawn by Snijders (1990) and Clement and Parry (1998). These authors working on winter wheat also analysed fungal colonization and disease symptoms induced by F. culmorum, F. graminaerum and M. nivale using scanning electron microscopy. They documented systemic growth of analysed fungi from the host stem-base to the head. Microscopic analysis displayed that all three fungal species colonized the spaces between successive leaf sheaths. Infection was the largest between the outer sheaths at proximity of the soil level and decreased both, above this region and inwards towards the culms. Growth within the tissues occurred from the inner epidermal surface of the outer leaf sheath. Hyphae penetrated and/or emerged along the line of the anticline walls between epidermal cells or entered directly through the epidermal cell wall.
DAB staining of both non-hardened and hardened triticale seedlings revealed H2O2 generation in the stomata apparatus as well as in leaf mesophyl cells. This observation confirmed the results from the fluorescence microscopy showing the hyphae penetration into leaf tissues occurring via stomata apparatuses. The differences in H2O2 level of accumulation between model cultivars in DAB stained sites were hardly visible, but our previous research confirmed the increased activity of antioxidative enzymes and the resulting lower H2O2 concentration in leaves was positively correlated with resistance to low temperature, tolerance to low illumination and resistance to pink snow mould infection in winter triticale seedlings (Golebiowska et al. 2010).
Hydrogen peroxide was often reported as involved in defence reactions against pathogens (Peng and Kuć 1992; Thordal-Christensen et al. 1997; Van Camp et al. 1998; Dat et al. 2000). Beside a direct antimicrobial effect (review by Heath 2002), H2O2 stimulates peroxysomes, activates defence genes, and stimulates numerous modifications of plant cell walls, i.e. oxidative cross-linking of phenolic compounds in the process of lignification (Baker and Orlandi 1995; Hammond-Kossack and Jones 1996; Mellersh et al. 2002). As phenolic compound accumulation was detected in our study at the site of pathogenic hyphae penetration, this reaction seems to belong to triticale defense system against M. nivale. However, high and unbalanced H2O2 levels may cause toxic effects on plants and even fasten pathogenesis of M. nivale, as our previous research shown (Golebiowska et al. 2010).
Our cytological observation did not show any signs of root damages. We found that M. nivale hyphae were present in the neighbourhood of roots and root hairs of non-hardened plants on the 7th day post-infection. These data are in accordance with data of Clement and Parry (1998), who did not observe either penetration of root cells or damage of the root system caused by M. nivale.
To our knowledge, this work presents the first observation of M. nivale haustoria projected into stomata apparatus and cortex cells of cereals crowns. Similar observations on Festulolium perenne were reported by Dubas et al. (2010). Moreover, our results show that the leaf sheath around crown is a particularly sensitive infection area and therefore the cytological differences of these tissues might be important in early defence reaction.
Abbreviations
- ROS:
-
Reactive oxygen species
- DAB:
-
3,3′-Diaminobenzidine
- LTB:
-
Low temperature basidiomycetes
- PAR:
-
Photosynthetically active radiation
References
Antikainen M, Griffith M, Zhang J, Hon WC, Yang DSC, Pihakaski-Maunsbach K (1996) Immunolocalization of antifreeze proteins in winter rye leaves, crowns and roots by tissue printing. Plant Physiol 110:845–857
Baker CJ, Orlandi EW (1995) Active oxygen in plant pathogenesis. Ann Rev Phytopathol 33:299–321
Browne RA, Mascher F, Golebiowska G, Hofgaard IS (2006) Components of partial disease resistance in wheat detected in a detached leaf assay inoculated with Microdochium majus using first, second and third expanding seedling leaves. J Phytopathol 154(5):204–208
Cichy H, Maćkowiak W (1993) Intravarietal differences in winter triticale resistance to snow mould - Fusarium nivale. Plant Breed Acclim Seed Sci 37(3):115–119
Clement JA, Parry DW (1998) Stem-base disease and fungal colonization of winter wheat grown in compost inoculated with Fusarium culmorum, F. graminearum and Microdochium nivale. Eu J Plant Pathol 104:323–330
Cormack MW, Lebeau JB (1959) Snow mold infection of Alfalfa, grasses, and winter wheat by several fungi under artificial conditions. Botany 37:685–693
Dat JS, Vandenabeele E, Vranová E, Van Montagu M, Inzé D, Van Breusegen F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
Dubas E, Marzec K, Płażek A (2010) Cytological studies on Microdochium nivale infection in Lolium perenne (L.) plants. Adv Agr Sci Problem Issues 545 (in press)
Ergon Å, Tronsmo AM (2006) Components of pink snow mould resistance in winter wheat are expressed prior to cold hardening and in detached leaves. J Phytopathol 154(3):134–142
Ergon A, Klemsdal SS, Tronsmo AM (1998) Interactions between cold hardening and Microdochium nivale infection on expression of pathogenesis-related genes in winter wheat. Physiol Mol Plant Pathol 53:301–310
Gaudet DA, Kokko EG (1985) Penetration and infection of winter wheat leaves by Coprinus psychromorbidus under controlled environment conditions. Botany 63(5):955–960
Gaudet DA, Laroche A, Frick M, Davoren J, Puchalski B, Ergon A (2000) Expression of plant defence-related (PR-proteins) transcripts during hardening and dehardening of winter wheat. Physiol Mol Plant Pathol 57:15–24
Golebiowska G, Wedzony M (2009) Cold-hardening of winter triticale (×Triticosecale Wittm.) results in increased resistance to pink snow mould Microdochium nivale (Fr., Samuels & Hallett) and genotype-dependent chlorophyll fluorescence modulations. Acta Physiol Plant 31(6):12–19
Golebiowska G, Wedzony M, Plazek A (2010) The responses of pro- and antioxidative systems to cold-hardening and pathogenesis differs in triticale (×Triticosecale Wittm.) seedlings susceptible or resistant to pink snow mould (Microdochium nivale Fr., Samuels & Hallett). J Phytopahol (in press)
Hammond-Kossack KE, Jones JDG (1996) Resistance gene-dependent plant defence responses. Plant Cell 8:1773–1791
Heath MC (2002) Cellular interactions between biotrophic fungal pathogens and host or nonhost plants. Can J Plant Pathol 24:259–264
Hiilovaara-Teijo M, Hannukkala A, Griffith M, Yu XM, Pihakaski-Maunsbach K (1999) Snow-mold-induced apoplastic proteins in winter rye leaves lack antifreeze activity. Plant Physiol 121:665–673
Hincha DK, Meins JF, Schmitt JM (1997) β-1, 3-Glucanase is cryoprotective in vitro and is accumulated in leaves during cold acclimation. Plant Physiol 114:1077–1083
Jackowiak H, Packa D, Wiwart M, Perkowski J (2005) Scanning electron microscopy of Fusarium damaged kernels of spring wheat. Int J Food Microbiol 98(2):113–123
Kang Z, Buchenauer H, Huang L, Han Q, Zhang H (2007) Cytological and immunocytochemical studies on responses of wheat spikes of the resistant Chinese cv. Sumai 3 and the susceptible cv. Xiaoyan 22 to infection by Fusarium graminearum. Eur J Plant Phatol 120(4):383–396
Koczowska I, Packa D (1986) Anatomo-physiological resistance of rye plants to infection by Fusarium nivale (Fr.) Cs. Acta Acad Agric Technol Olsztyn 43:129–142
Kumar J, Schäfer P, Hückelhoven R, Langen G, Baltruschat H, Stein E, Nagarajan S, Kogel KH (2002) Bipolaris sorokiniana, a cereal pathogen of global concern: cytological and molecular approaches towards better control. Mol Plant Pathol 3(4):185–195
Kuzniak E, Urbanek H (2000) The involvement of hydrogen peroxide in plant responses to stresses. Acta Physiol Plantarum 22(2):195–203
Mellersh DG, Foulds IV, Higgins VJ, Heath MC (2002) H2O2 plays different roles in determining penetration failure in three diverse plant–fungal interactions. Plant J 29:257–268
Mlodzianowski F, Wozny A (eds) (1990) Wykłady i ćwiczenia z biologii komórki roślinnej, ver. III. University of Poznan Press, Poland
Nakajima T, Abe J (1996) Environmental factors affecting expression of resistance to pink snow mould caused by Microdochium nivale in winter wheat. Can J Bot 74:1783–1788
O’Brien TP, McCully ME (eds) (1981) The study of plant structure. Principles and selected methods. Termarcarphi Pty. Ltd. Melbourne Press, Australia
Oren L, Ezrati S, Cohen D, Sharon A (2003) Early events in the Fusarium verticillioides-maize interaction characterized by using a Green Fluorescent Protein-expressing transgenic isolate. Appl Environ Microbiol 69:1695–1701
Peng M, Kuć J (1992) Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disks. Phytopathology 82:696–699
Pitzschke A, Hirt H (2006) Mitogen-activated protein kinases and reactive oxygen species signaling in plants. Plant Physiol 141:351–356
Pronczuk M, Madej L, Kolasinska I (2003) Research for resistance to Microdochium nivale among inbred lines of rye. Plant Breed Seed Sci 48(2):83–86
Ryöppy PH (1997) Haploidy in triticale. In: Mohan Jain S, Sopory SK, Veilleux RE (eds) In vitro haploid production in higher plants: cereals, vol 4, no. 5. Kluwer, Dordrecht, pp 117–131
Snijders CHA (1990) Systemic fungal growth of Fusarium culmorum in stems of winter wheat. J Phytopathol 129:133–140
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
Tronsmo AM, Gregersen P, Hjeljord L, Sandal T, Bryngelsson T, Collinge DB (1993) Cold-induced disease resistance. In: Fritig B, Legrand M (eds) Mechanisms of plant defense. Kluwer, Dordrecht
Tronsmo AM, Hsiang T, Okuyama H, Nakajima T (2001) Low temperature diseases caused by Microdochium nivale. In: Iriki N, Gaudet DA, Tronsmo AM, Matsumoto N, Yoshida M, Nishimune A (eds) Low temperature plant microbe interactions under snow. Hokkaido National Agricultural Experiment Station, Sapporo, pp 75–86
Van Camp W, Van Montagu M, Inze D (1998) H2O2 and NO: redox signals in disease resistance. Trends Plant Sci 3:330–334
Acknowledgments
This work was supported by the research project MNiSW 7/COS/2007/01 supporting COST Action 860 SUSVAR.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Barna.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Dubas, E., Golebiowska, G., Zur, I. et al. Microdochium nivale (Fr., Samuels & Hallett): cytological analysis of the infection process in triticale (×Triticosecale Wittm.). Acta Physiol Plant 33, 529–537 (2011). https://doi.org/10.1007/s11738-010-0576-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0576-9