Abstract
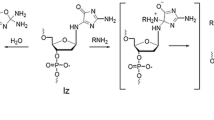
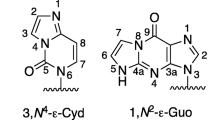
Biological application of conjugates derived from oligonucleotides and quinone methides have previously been limited by the slow exchange of their covalent self-adducts and subsequent alkylation of target nucleic acids. To enhance the rates of these processes, a new quinone methide precursor with an electron donating substituent has been prepared. Additionally, this substituent has been placed para to the nascent exo-methylene group of the quinone methide for maximum effect. A conjugate made from this precursor and a 5'-aminohexyloligonucleotide accelerates formation of its reversible self-adduct and alkylation of its complementary DNA as predicted from prior model studies.

Similar content being viewed by others
References
Rokita S E, ed. Quinone Methides. Hoboken: John Wiley & Sons, Inc., 2009, 1–431
Shabat D, Shamis M. Single-triggered AB6 self-immolative dendritic amplifiers. Chemistry (Weinheim an der Bergstrasse, Germany), 2007, 13: 4523–4528
Rosenau T, Kloser E, Gille L, Mazzini F, Netscher T. Vitamin E chemistry. Studies into initial oxidation intermediates of a-tocopherol: Disproving the involvement of 5a-C-centered “chromanol methide” radicals. Journal of Organic Chemistry, 2007, 72: 3268–3281
Colloredo-Mels S, Dorr R T, Verga D, Freccero M. Photogenerated quinone methides as useful intermediates in the synthesis of chiral BINOL ligands. Journal of Organic Chemistry, 2006, 71: 3889–3895
Arumugam P, Popik V V. Attach, remove, or replace: Reversible surface functionalization using thiol-quinone methide photoclick chemistry. Journal of the American Chemical Society, 2012, 134: 8408–8411
Hulsman N, Medema J P, Bos C, Jongejan A, Luers R, Smit M J, de Esch I J P, Richel D, Witjmans M. Chemical insights in the concept of hybrid drugs: The antitumor effect of nitric oxide-donating aspirin involves a quinone methide but not nitric oxide nor aspirin. Journal of Medicinal Chemistry, 2007, 50: 2424–2431
Kashfi K, Rigas B. The mechanism of action of nitric oxidedonating aspirin. Biochemical and Biophysical Research Communications, 2007, 358: 1096–1101
Dunlap T, Chandrasena R E P, Wang Z, Sinha V, Wang Z, Thatcher G R J. Quinone formation as a chemoprevention strategy for hybrid drugs: Balancing cytotoxity and cytoprotection. Chemical Research in Toxicology, 2007, 20: 1903–1912
Lewis M A, Yoerg D G, Bolton J L, Thompson J. Alkylation of 2'-deoxynucleosides and DNA by quinone methides derived from 2,6-di-tert-butyl-4-methylphenol. Chemical Research in Toxicology, 1996, 9: 1368–1374
Weinert E E, Frankenfield K N, Rokita S E. Time-dependent evolution of adducts formed between deoxynucleosides and a model quinone methide. Chemical Research in Toxicology, 2005, 18: 1364–1370
McCrane M P, Hutchinson M, Ad O, Rokita S E. Oxidative quenching of quinone methide adducts reveals transient products of reversible alkylation in duplex DNA. Chemical Research in Toxicology, 2014, 27: 1282–1293
Lönnberg T, Hutchinson M, Rokita S E. Selective alkylation of Crich bulge motifs in nucleic acids by quinone methide derivatives. Chemistry, 2015, 21: 13127–13186
Modica E, Zanaletti R, Freccero M, Mella M. Alkylation of amino acids and glutathione in water by ortho-quinone methides. Reactivity and selectivity. Journal of Organic Chemistry, 2001, 66: 41–52
Zhou Q, Zuniga M A. Quinone methide formations in the Cu2+-induced oxidation of a diterpenone cataechol and concurrent damage on DNA. Chemical Research in Toxicology, 2005, 18: 382–388
Wang P, Liu R, Wu X, Ma H, Cao X, Zhou P, Zhang J, Weng X, Zhang X L, Zhou X, Weng L A. Potent, water-soluble and photoinducible DNA cross-linking agent. Journal of the American Chemical Society, 2003, 125: 1116–1117
Percivalle C, La Rosa A, Verga D, Doria F, Mella M, Palumbo M, Di Antonio M, Freccero M. Quinone methide generation via photoinduced electron transfer. Journal of Organic Chemistry, 2011, 76: 3096–3106
Myers J K, Widlanski T S. Mechanism-based inactivation of prostatic acid phosphatase. Science, 1993, 262: 1451–1453
Kwan D H, Chen H M, Ratananikom K, Hancock S M, Watanabe Y, Kongsaeree P T, Samuels A L, Withers S G. Self-immobilizing fluorogenic imaging agents of enzyme activity. Angewandte Chemie International Edition, 2011, 50: 300–303
Cao S, Wang Y, Peng X. ROS-inducible DNA cross-linking agent as a new anticancer prodrug building block. Chemistry, 2012, 18: 3850–3854
Dufrasne F, Gelbcke M, Néve J, Kiss R, Kraus J L. Quinone methides and their prodrugs: A subtle equilibrium between cancer promotion, prevention and cure. Current Medicinal Chemistry, 2011, 18: 3995–4011
Toteva M M, Richard J P. The generation and reactions of quinone methides. Advances in Physical Organic Chemistry, 2011, 45: 39–91
Cao S, Peng X. Exploiting endogenous cellular process to generate quinone methides in vivo. Current Organic Chemistry, 2014, 18: 70–85
Bolton J L. Quinone methide bioactivation pathway: Contribution to toxicity and/or cytoprotection. Current Organic Chemistry, 2014, 18: 61–69
Percivalle C, Doria F, Freccerro M. Quinone methides as DNA alkylating agents: An overview on efficient activation protocols for enhanced target selectivity. Current Organic Chemistry, 2014, 18: 19–43
Weinert E E, Dondi R, Colloredo-Mels S, Frankenfield K N, Mitchell C H, Freccero M, Rokita S E. Substituents on quinone methides strongly modulate formation and stability of their nucleophilic adducts. Journal of the American Chemical Society, 2006, 128: 11940–11947
Cao S, Christiansen R, Peng X. Substituent effects on oxidationinduced formation of quinone methides from arylboronic ester precursors. Chemistry, 2013, 19: 9050–9058
Veldhuyzen W F, Pande P, Rokita S E. A transient product of DNA alkylation can be stabilized by binding localization. Journal of the American Chemical Society, 2003, 125: 14005–14013
Kumar D, Veldhuyzen W F, Zhou Q, Rokita S E. Conjugation of a hairpin pyrrole-imidazole polyamide to a quinone methide for control of DNA cross-linking. Bioconjugate Chemistry, 2004, 15: 915–922
Li T, Rokita S E. Selective modification of DNA controlled by an ionic signal. Journal of the American Chemical Society, 1991, 113: 7771–7773
Liu Y, Rokita S E. Inducible alkylation of DNA by a quinone methide-peptide nucleic acid conjugate. Biochemistry, 2012, 51: 1020–1027
Zhou Q, Rokita S E. A general strategy for target-promoted alkylation in biological systems. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100: 15452–15457
Wang H. Quinone methides and their biopolymer conjugates as reversible DNA alkylating agents. Current Organic Chemistry, 2014, 18: 44–60
Rossiter C S, Kumar D, Modica E, Rokita S E. Few constraints limit the design of quinone methide-oligonucleotide self-adducts for directing DNA alkylation. Chemical Communications, 2011, 46: 1476–1478
Zhou Q, Pande P, Johnson A E, Rokita S E. Sequence-specific delivery of a quinone methide intermediate to the major groove of DNA. Bioorganic & Medicinal Chemistry, 2001, 9: 2347–2354
Fakhari F, Rokita S E. A walk along DNA using bipedal migration of a dynamic and covalent cross-linker. Nature Communications, 2014, 5: 5591
Bolton J L, Sevestre H, Ibe B O, Thompson J A. Formation and reactivity of alternative quinone methides from butylated hydroxytoluene: Possible explanation for species-specific pneumotoxicity. Chemical Research in Toxicology, 1990, 3: 65–70
Wang H, Wahi M S, Rokita S E. Immortalizing a transient electrophile for DNA cross-linking. Angewandte Chemie International Edition, 2008, 47: 1291–1293
Wang H, Rokita S E. Dynamic cross-linking is retained in duplex DNA after multiple exchange of strands. Angewandte Chemie International Edition, 2010, 49: 5957–5960
Laganis E D, Chenard B L. Metal silanolates: Organic soluble equivalents for O–2. Tetrahedron Letters, 1984, 25: 5831–5834
Shirali A, Sriram M, Hall J J, Nguyen B L, Guddneppanavar R, HadimaniMB, Ackley J F, Siles R, Jelinek C J, Arthasery P, Brown R C, Murrell V L. MeModie A, Sharma S, Chaplin D J, Pinney K G. Development of synthetic methodology suitable for the radiosynthesis of combretastatin A-1 (CA1) and its corresponding prodrug CA1P. Journal of Natural Products, 2009, 72: 414–421
Mangas-Sánchez J, Rodriguez-Mata M, Busto E, Gotor-Fernández V, Gotor V. Chemoenzymatic synthesis of rivastigmine based on lipase-catalyzed processes. Journal of Organic Chemistry, 2009, 74: 5304–5310
Rokita S E. Reversible alkylation of DNA by quinone methides. Hoboken: Wiley, 2009, 297–327
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Huang, C., Rokita, S.E. DNA alkylation promoted by an electron-rich quinone methide intermediate. Front. Chem. Sci. Eng. 10, 213–221 (2016). https://doi.org/10.1007/s11705-015-1541-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-015-1541-3