Abstract
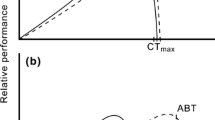
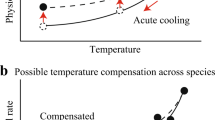
A continuing issue in evolutionary thermal biology is the mismatch between preferred body temperatures (T pref) and optimal temperatures (T opt) for whole-animal performance. Using phylogenetic comparative analyses, I examined the hypothesis that a difference in the rates at which T pref and T opt evolve causes the mismatch in a lineage of European newts. In a laboratory thermal gradient, newts maintained body temperatures that were on average 8 °C below T opt for maximum swimming velocity. The lower boundary of the T pref range evolved faster than the mean T pref, the upper boundary of the T pref range, and T opt. The strong evolutionary co-variation between mean T pref and its boundaries prevented the shift of mean T pref away from T opt. This suggests that the variation in evolutionary rates has a limited potential to modify the disparity between thermal optima and preferenda.




Similar content being viewed by others
References
Ackerly, D. (2009). Conservatism and diversification of plant functional traits: Evolutionary rates versus phylogenetic signal. Proceedings of the National Academy of Sciences of the United States of America, 106, 19699–19706.
Adams, D. C. (2013). Comparing evolutionary rates for different phenotypic traits on a phylogeny using likelihood. Systematic Biology, 62, 181–192.
Angilletta, M. J., Bennett, A. F., Guderley, H., Navas, C. A., Seebacher, F., & Wilson, R. S. (2006). Coadaptation: A unifying principle in evolutionary thermal biology. Physiological and Biochemical Zoology, 79, 282–294.
Angilletta, M. J., Hill, T., & Robson, M. A. (2002a). Is physiological performance optimized by thermoregulatory behavior? A case study of the eastern fence lizard, Sceloporus undulatus. Journal of Thermal Biology, 27, 199–204.
Angilletta, M. J., Huey, R. B., & Frazier, M. R. (2010). Thermodynamic effects on organismal performance: Is hotter better? Physiological and Biochemical Zoology, 83, 197–206.
Angilletta, M. J., Niewiarowski, P. H., & Navas, C. A. (2002b). The evolution of thermal physiology in ectotherms. Journal of Thermal Biology, 27, 249–268.
Angilletta, M. J., Wilson, R. S., Navas, C. A., & James, R. S. (2003). Tradeoffs and the evolution of thermal reaction norms. Trends in Ecology & Evolution, 18, 234–240.
Arnold, S. J. (1992). Constraints on phenotypic evolution. American Naturalist, 140, S85–S107.
Asbury, D. A., & Angilletta, M. J. (2010). Thermodynamic effects on the evolution of performance curves. American Naturalist, 176, E40–E49.
Bauwens, D., Garland, T., Castilla, A. M., & Van Damme, R. (1995). Evolution of sprint speed in lacertid lizards: Morphological, physiological, and behavioral covariation. Evolution, 49, 848–863.
Beitinger, T. L., & Fitzpatrick, L. C. (1979). Physiological and ecological correlates of preferred temperature in fish. American Zoologist, 19, 319–329.
Bennett, A. F., & Licht, P. (1972). Anaerobic metabolism during activity in lizards. Journal of Comparative Physiology, 81, 277–288.
Boettiger, C., Coop, G., & Ralph, P. (2012). Is your phylogeny informative? Measuring the power of comparative methods. Evolution, 66, 2240–2251.
Castañeda, L. E., Lardies, M. A., & Bozinovic, F. (2004). Adaptive latitudinal shifts in the thermal physiology of a terrestrial isopod. Evolutionary Ecology Research, 6, 579–593.
Cossins, A. R., & Bowler, K. (1987). Temperature biology of animals. New York: Chapman and Hall.
Dawson, W. R. (1975). On the physiological significance of the preferred body temperatures of reptiles. In D. M. Gates & R. B. Schmerl (Eds.), Perspectives of biophysical ecology (pp. 443–473). New York: Springer.
Deere, J. A., & Chown, S. L. (2006). Testing the beneficial acclimation hypothesis and its alternatives for locomotor performance. American Naturalist, 168, 630–644.
DiCiccio, T. J., & Efron, B. (1996). Bootstrap confidence intervals. Statistical Science, 11, 189–212.
Dvořák, J., & Gvoždík, L. (2010). Adaptive accuracy of temperature oviposition preferences in newts. Evolutionary Ecology, 24, 1115–1127.
Felsenstein, J. (2008). Comparative methods with sampling error and within-species variation: Contrasts revisited and revised. American Naturalist, 171, 713–725.
Gilchrist, G. W. (1995). Specialists and generalists in changing environments. 1. Fitness landscapes of thermal sensitivity. American Naturalist, 146, 252–270.
Gilchrist, G. W. (1996). A quantitative genetic analysis of thermal sensitivity in the locomotor performance curve of Aphidius ervi. Evolution, 50, 1560–1572.
Gittleman, J. L., Anderson, C. G., Kot, M., & Luh, H.-K. (1996). Phylogenetic lability and rates of evolution: A comparison of behavioral, morphological and life history traits. In E. P. Martins (Ed.), Phylogenies and the comparative method in animal behavior (pp. 166–205). Oxford: Oxford University Press.
Griffiths, R. A. (1996). Newts and salamanders of Europe. London: Academic Press.
Gvoždík, L. (2003). Postprandial thermophily in the Danube crested newt, Triturus dobrogicus. Journal of Thermal Biology, 28, 545–550.
Gvoždík, L. (2005). Does reproduction influence temperature preferences in newts? Canadian Journal of Zoology, 83, 1038–1044.
Gvoždík, L., Puky, M., & Šugerková, M. (2007). Acclimation is beneficial at extreme test temperatures in the Danube crested newt, Triturus dobrogicus (Caudata, Salamandridae). Biological Journal of the Linnean Society, 90, 627–636.
Gvoždík, L., & Van Damme, R. (2008). The evolution of thermal performance curves in semi-aquatic newts: Thermal specialists on land and thermal generalists in water? Journal of Thermal Biology, 33, 395–403.
Hadamová, M., & Gvoždík, L. (2011). Seasonal acclimation of preferred body temperatures improves the opportunity for thermoregulation in newts. Physiological and Biochemical Zoology, 84, 166–174.
Hertz, P. E., Huey, R. B., & Stevenson, R. D. (1993). Evaluating temperature regulation by field-active ectotherms: The fallacy of the inappropriate question. American Naturalist, 142, 796–818.
Hochachka, P. W., & Somero, G. N. (2002). Biochemical adaptation: Mechanism and process in physiological evolution. Oxford: Oxford University Press.
Huey, R. B. (1982). Temperature, physiology, and the ecology of reptiles. In C. Gans & F. H. Pough (Eds.), Biology of the reptilia, Physiology C, Physiological ecology (Vol. 12, pp. 25–91). London: Academic Press.
Huey, R. B., & Bennett, A. F. (1987). Phylogenetic studies of coadaptation: Preferred temperatures versus optimal performance temperatures of lizards. Evolution, 41, 1098–1115.
Huey, R. B., & Bennett, A. F. (1990). Physiological adjustments to fluctuating thermal environments: An ecological and evolutionary perspective. In A. Morimoto, A. Tissieres, & C. Georgopoulus (Eds.), Stress proteins in biology and medicine (pp. 37–59). Spring: Cold Spring Harbor Laboratory Press.
Huey, R. B., Kearney, M. R., Krockenberger, A., Holtum, J. A. M., Jess, M. & Williams S. E. (2012). Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology, and adaptation. Philosophical Transactions of the Royal Society B, 367, 1665–1679.
Huey, R. B., & Stevenson, R. D. (1979). Integrating thermal physiology and ecology of ectotherms: A discussion of approaches. American Zoologist, 19, 357–366.
Johnston, I. A., & Temple, G. K. (2002). Thermal plasticity of skeletal muscle phenotype in ectothermic vertebrates and its significance for locomotory behaviour. Journal of Experimental Biology, 205, 2305–2322.
Kelsch, S. W., & Neill, W. H. (1990). Temperature preference vs. acclimation in fishes: Selection for changing metabolic optima. Transactions of the American Fisheries Society, 119, 601–610.
Kurdíková, V., Smolinský, R., & Gvoždík, L. (2011). Mothers matter too: Benefits of temperature oviposition preferences in newts. PLoS ONE, 6, e23842.
Marek, V., & Gvoždík, L. (2012). The insensitivity of thermal preferences to various thermal gradient profiles in newts. Journal of Ethology, 30, 35–41.
Martin, T. L., & Huey, R. B. (2008). Why “suboptimal” is optimal: Jensen’s inequality and ectotherm thermal preferences. American Naturalist, 171, E102–E118.
Muñoz, M. M., Stimola, M. A., Algar, A. C., Conover, A., Rodriguez, A. J., Landestoy, M. A., et al. (2014). Evolutionary stasis and lability in thermal physiology in a group of tropical lizards. Proceedings of the Royal Society B, 281, 20132433.
O’Meara, B. C., Ane, C., Sanderson, M. J., & Wainwright, P. C. (2006). Testing for different rates of continuous trait evolution using likelihood. Evolution, 60, 922–933.
Paradis, E. (2006). Analysis of phylogenetics and evolution with R. New York: Springer.
Pitchers, W., Wolf, J. B., Tregenza, T., Hunt, J., & Dworkin, I. (2014). Evolutionary rates for multivariate traits: The role of selection and genetic variation. Philosophical Transactions of the Royal Society B, 369, 20130252.
Pörtner, H. O. (2001). Climate change and temperature-dependent biogeography: Oxygen limitation of thermal tolerance in animals. Naturwissenschaften, 88, 137–146.
Revell, L. J. (2012). Phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223.
Šamajová, P., & Gvoždík, L. (2009). The influence of temperature on diving behaviour in the alpine newt, Triturus alpestris. Journal of Thermal Biology, 34, 401–405.
Smolinský, R., & Gvoždík, L. (2009). The ontogenetic shift in thermoregulatory behaviour of newt larvae: Testing the ‘enemy-free temperatures’ hypothesis. Journal of Zoology, 279, 180–186.
Somero, G. N., Dahlhoff, E., & Lin, J. J. (1996). Stenotherms and eurytherms: Mechanisms establishing thermal optima and tolerance ranges. In I. A. Johnston & A. F. Bennett (Eds.), Animals and temperature: Phenotypic and evolutionary adaptation (pp. 53–78). Cambridge: Cambridge University Press.
Stevenson, R. D., Peterson, C. R., & Tsuji, J. S. (1985). The thermal dependence of locomotion, tongue flicking, digestion, and oxygen consumption in the wandering garter snake. Physiological Zoology, 58, 46–57.
Van Damme, R., Bauwens, D., & Verheyen, R. F. (1991). The thermal dependence of feeding behaviour, food consumption and gut-passage time in the lizard Lacerta vivipara Jacquin. Functional Ecology, 5, 507–517.
Venables, W. N., & Ripley, B. D. (2002). Modern applied statistics with S. New York: Springer.
Vieites, D. R., Nieto-Roman, S., & Wake, D. B. (2009). Reconstruction of the climate envelopes of salamanders and their evolution through time. Proceedings of the National Academy of Sciences of the United States of America, 106, 19715–19722.
Vinšálková, T., & Gvoždík, L. (2007). Mismatch between temperature preferences and morphology in F1 hybrid newts (Triturus carnifex × T. dobrogicus). Journal of Thermal Biology, 32, 433–439.
Wiens, J. J., Sparreboom, M., & Arntzen, J. W. (2011). Crest evolution in newts: Implications for reconstruction methods, sexual selection, phenotypic plasticity and the origin of novelties. Journal of Evolutionary Biology, 24, 2073–2086.
Williams, S. E., Shoo, L. P., Isaac, J. L., Hoffmann, A. A., & Langham, G. (2008). Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biology, 6, 2621–2626.
Yamamoto, A. H. (1994). Temperature preference of Drosophila immigrans and D. virilis: Intra- and inter-population genetic variation. Japanese Journal of Genetics, 69, 67–76.
Acknowledgments
I thank the anonymous reviewers for their comments on the previous versions of this manuscript, R. Van Damme for a detailed presubmission review, and D. Adams for help with his R script. This study was funded by a grant from the Czech Science Foundation (P506/10/2170) and institutional support (RVO: 68081766).
Conflict of interest
The author declares that he has no conflicts of interest.
Ethical standard
The author declares that this paper complies with the current laws of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gvoždík, L. Mismatch Between Ectotherm Thermal Preferenda and Optima for Swimming: A Test of the Evolutionary Pace Hypothesis. Evol Biol 42, 137–145 (2015). https://doi.org/10.1007/s11692-015-9305-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-015-9305-z