Abstract
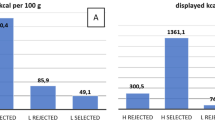
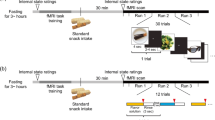
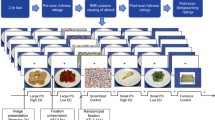
The extent that neural responsiveness to visual food stimuli is influenced by time of day is not well examined. Using a crossover design, 15 healthy women were scanned using fMRI while presented with low- and high-energy pictures of food, once in the morning (6:30–8:30 am) and once in the evening (5:00–7:00 pm). Diets were identical on both days of the fMRI scans and were verified using weighed food records. Visual analog scales were used to record subjective perception of hunger and preoccupation with food prior to each fMRI scan. Six areas of the brain showed lower activation in the evening to both high- and low-energy foods, including structures in reward pathways (P < 0.05). Nine brain regions showed significantly higher activation for high-energy foods compared to low-energy foods (P < 0.05). High-energy food stimuli tended to produce greater fMRI responses than low-energy food stimuli in specific areas of the brain, regardless of time of day. However, evening scans showed a lower response to both low- and high-energy food pictures in some areas of the brain. Subjectively, participants reported no difference in hunger by time of day (F = 1.84, P = 0.19), but reported they could eat more (F = 4.83, P = 0.04) and were more preoccupied with thoughts of food (F = 5.51, P = 0.03) in the evening compared to the morning. These data underscore the role that time of day may have on neural responses to food stimuli. These results may also have clinical implications for fMRI measurement in order to prevent a time of day bias.




Similar content being viewed by others
References
Allison, K. C., Goel, N., & Ahima, R. S. (2014). Delayed Timing of Eating: Impact on Weight and Metabolism. Current Obesity Reports , 3, 91–100.
Avants, B., Duda, J. T., Kim, J., Zhang, H., Pluta, J., Gee, J. C., & Whyte, J. (2008). Multivariate analysis of structural and diffusion imaging in traumatic brain injury. Academic Radiology, 15(11), 1360–1375. doi:10.1016/j.acra.2008.07.007.
Beechy, L., Galpern, J., Petrone, A., & Das, S. K. (2012). Assessment tools in obesity - psychological measures, diet, activity, and body composition. Physiology & Behavior, 107(1), 154–171. doi:10.1016/j.physbeh.2012.04.013.
Brignell, C., Griffiths, T., Bradley, B. P., & Mogg, K. (2009). Attentional and approach biases for pictorial food cues. Influence of external eating. Appetite, 52(2), 299–306. doi:10.1016/j.appet.2008.10.007.
Bromberg-Martin, E. S., Matsumoto, M., & Hikosaka, O. (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron, 68(5), 815–834. doi:10.1016/j.neuron.2010.11.022.
Bruce, A. S., Lepping, R. J., Bruce, J. M., Cherry, J. B., Martin, L. E., Davis, A. M., & Savage, C. R. (2013). Brain responses to food logos in obese and healthy weight children. Journal of Pediatrics, 162(4), 759–764. doi:10.1016/j.jpeds.2012.10.003. e752.
Conway, J. M., Ingwersen, L. A., Vinyard, B. T., & Moshfegh, A. J. (2003). Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. American Journal of Clinical Nutrition, 77(5), 1171–1178.
Conway, J. M., Ingwersen, L. A., & Moshfegh, A. J. (2004). Accuracy of dietary recall using the USDA five-step multiple-pass method in men: An observational validation study. Journal of the American Dietetic Association, 104(4), 595–603. doi:10.1016/j.jada.2004.01.007.
Cox, R. W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173.
de Castro, J. M. (2004). The time of day of food intake influences overall intake in humans. The Journal of Nutrition, 134(1), 104–111.
Ernst, M., & Vingiano, W. (1989). Development of a graphic psychiatric self-rating scale. Comprehensive Psychiatry, 30(2), 189–194.
Fedoroff, I. C., Polivy, J., & Herman, C. P. (1997). The effect of pre-exposure to food cues on the eating behavior of restrained and unrestrained eaters. Appetite, 28(1), 33–47.
Fedoroff, I., Polivy, J., & Herman, C. P. (2003). The specificity of restrained versus unrestrained eaters' responses to food cues: general desire to eat, or craving for the cued food? Appetite, 41(1), 7–13.
Hasler, B. P., Forbes, E. E., & Franzen, P. L. (2014). Time-of-day differences and short-term stability of the neural response to monetary reward: a pilot study. Psychiatry Research, 224(1), 22–27. doi:10.1016/j.pscychresns.2014.07.005.
Holsen, L. M., Savage, C. R., Martin, L. E., Bruce, A. S., Lepping, R. J., Ko, E., & Goldstein, J. M. (2012). Importance of reward and prefrontal circuitry in hunger and satiety: Prader-Willi syndrome vs simple obesity. International Journal of Obesity, 36(5), 638–647. doi:10.1038/ijo.2011.204.
Killgore, W. D., Young, A. D., Femia, L. A., Bogorodzki, P., Rogowska, J., & Yurgelun-Todd, D. A. (2003). Cortical and limbic activation during viewing of high- versus low-calorie foods. NeuroImage, 19(4), 1381–1394.
Lacy, J. W., Yassa, M. A., Stark, S. M., Muftuler, L. T., & Stark, C. E. (2011). Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learning & Memory, 18(1), 15–18. doi:10.1101/lm.1971111.
LeCheminant, J. D., Christenson, E., Bailey, B. W., & Tucker, L. A. (2013). Restricting night-time eating reduces daily energy intake in healthy young men: a short-term cross-over study. British Journal of Nutrition, 1–6. doi:10.1017/S0007114513001359
Lumeng, J. C., & Burke, L. M. (2006). Maternal prompts to eat, child compliance, and mother and child weight status. Journal of Pediatrics, 149(3), 330–335. doi:10.1016/j.jpeds.2006.04.009.
McGonigle, D. J. (2012). Test-retest reliability in fMRI: Or how I learned to stop worrying and love the variability. NeuroImage, 62(2), 1116–1120. doi:10.1016/j.neuroimage.2012.01.023.
Motley, S. E., & Kirwan, C. B. (2012). A parametric investigation of pattern separation processes in the medial temporal lobe. Journal of Neuroscience, 32(38), 13076–13085. doi:10.1523/JNEUROSCI. 5920-11.2012.
Muranishi, M., Inokawa, H., Yamada, H., Ueda, Y., Matsumoto, N., Nakagawa, M., & Kimura, M. (2011). Inactivation of the putamen selectively impairs reward history-based action selection. Experimental Brain Research, 209(2), 235–246. doi:10.1007/s00221-011-2545-y.
Murdaugh, D. L., Cox, J. E., Cook, E. W., 3rd, & Weller, R. E. (2012). fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. NeuroImage, 59(3), 2709–2721.
Ng, J., Stice, E., Yokum, S., & Bohon, C. (2011). An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite, 57(1), 65–72. doi:10.1016/j.appet.2011.03.017.
Ogden, C. L., Carroll, M. D., Kit, B. K., & Flegal, K. M. (2014). Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA, 311(8), 806–814. doi:10.1001/jama.2014.732.
Prinsen, S., de Ridder, D. T., & de Vet, E. (2013). Eating by example. Effects of environmental cues on dietary decisions. Appetite, 70, 1–5. doi:10.1016/j.appet.2013.05.023.
Probst, Y. C., & Tapsell, L. C. (2005). Overview of computerized dietary assessment programs for research and practice in nutrition education. Journal of Nutrition Education and Behavior, 37(1), 20–26.
Rolls, E. T. (1999). The brain and emotion. Oxford: Oxford University Press.
Rothemund, Y., Preuschhof, C., Bohner, G., Bauknecht, H. C., Klingebiel, R., Flor, H., & Klapp, B. F. (2007). Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage, 37(2), 410–421. doi:10.1016/j.neuroimage.2007.05.008.
Stunkard, A. J., Allison, K. C., Geliebter, A., Lundgren, J. D., Gluck, M. E., & O'Reardon, J. P. (2009). Development of criteria for a diagnosis: lessons from the night eating syndrome. Comprehensive Psychiatry, 50(5), 391–399. doi:10.1016/j.comppsych.2008.09.013.
Sweet, L. H., Hassenstab, J. J., McCaffery, J. M., Raynor, H. A., Bond, D. S., Demos, K. E., & Wing, R. R. (2012). Brain response to food stimulation in obese, normal weight, and successful weight loss maintainers. Obesity (Silver Spring), 20(11), 2220–2225. doi:10.1038/oby.2012.125.
Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system : an approach to cerebral imaging. Stuttgart: Georg Thieme.
Toombs, R. J., Ducher, G., Shepherd, J. A., & De Souza, M. J. (2012). The impact of recent technological advances on the trueness and precision of DXA to assess body composition. Obesity (Silver Spring), 20(1), 30–39. doi:10.1038/oby.2011.211.
Yassa, M. A., Lacy, J. W., Stark, S. M., Albert, M. S., Gallagher, M., & Stark, C. E. (2011). Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus, 21(9), 968–979. doi:10.1002/hipo.20808.
Yokum, S., Ng, J., & Stice, E. (2011). Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring), 19(9), 1775–1783. doi:10.1038/oby.2011.168.
Acknowledgments
TDM, CBK, LED, and JDL designed research; TDM and CBK conducted research; TDM, CBK, and JDL analyzed data; TDM, CBK, LED and JDL wrote the paper; TDM had primary responsibility for final content. All authors read and approved the final manuscript.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
Conflict of interest
Travis Masterson, James D. LeCheminant, C. Brock Kirwan, and Lance E. Davidson state that they have no conflict of interested associated with this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
(PDF 68 kb)
Rights and permissions
About this article
Cite this article
Masterson, T.D., Kirwan, C.B., Davidson, L.E. et al. Neural reactivity to visual food stimuli is reduced in some areas of the brain during evening hours compared to morning hours: an fMRI study in women. Brain Imaging and Behavior 10, 68–78 (2016). https://doi.org/10.1007/s11682-015-9366-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-015-9366-8