Abstract
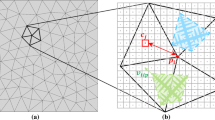
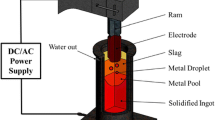
The electroslag remelting (ESR) process is a widely used secondary remelting process for the production of high-value-added alloys and steels. The grain structure of ESR ingot has a great effect on the final properties of products. A multiscale mathematical model combining the macroscopic heat transport with the mesoscopic nucleation and grain growth was developed to predict the grain structure evolution of solidification ingot during the ESR process. A moving cell frame, which dynamically defines the calculation domain for grain structure simulation, was proposed to save the computation resources and time. The thermophysical properties of steel related to the solidification of rotor steel 30Cr1Mo1V were adopted in present model and the nucleation parameters, which were suitable for the ESR process, were determined using the trial and error method in numerical simulation. The multiscale mathematical model was validated by the comparison between predicted and experimentally observed grain structure, and the results showed that the model was capable of simulating the grain structure evolution during the ESR process. Finally, the preliminary investigation on the effect of industrial process parameters on the grain structure was carried out and the results showed that increasing melting rate caused finer columnar grain structure and changed the growth direction of columnar grain structure from the axial–radial growth into the radial growth at very high melting rate. Meanwhile, increasing the molten slag temperature made the columnar grain structure finer and reduced the thickness of the refined equiaxed grain layer both at the surface and bottom of the ESR ingot.













Similar content being viewed by others
Abbreviations
- a 0 :
-
Interatomic distance (m)
- A m :
-
Cross-sectional area of mold (m2)
- Ar 4 :
-
δ/γ Phase transformation temperature (°C)
- C p :
-
Specific heat (J kg−1 K−1)
- C 0 :
-
Solute concentration (wt pct)
- C R :
-
Cooling rate (°C s−1)
- D :
-
Solute diffusion coefficient in liquid
- f s :
-
Solid fraction (–)
- G :
-
Interface temperature gradient (°C m−1)
- G c :
-
Interface concentration gradient (wt pct m−1)
- h sm, h mm, h mb :
-
Heat transfer coefficients of slag/melt interface, melt/mold interface, melt/base plate interface (W m−2 K−1)
- k, k v :
-
Equilibrium partition coefficient, nonequilibrium partition coefficient (–)
- T, T slag, T w :
-
Temperature, average temperature of slag pool, temperature of cooling water (°C)
- L :
-
Latent heat of solidification (J kg−1)
- M e :
-
Melting rate (kg s−1)
- m, m v :
-
Equilibrium liquidus slope, nonequilibrium liquidus slope (°C/%)
- n :
-
δ/γ Phase transformation line slope (°C/%)
- n max :
-
Maximum nuclei density (–)
- n(∆T), δn :
-
Grain density, increase of grain density (–)
- n sc(Δt):
-
New solidified cell number (–)
- n tc :
-
Total cell number (–)
- P C :
-
Solute Péclet number (–)
- R :
-
Dendrite tip radius (m)
- t :
-
Time (s)
- V m :
-
Growth velocity of solidifying ingot (m s−1)
- V, V max :
-
Dendritic tip growth velocity, maximum dendritic tip growth velocity (m s−1)
- w c :
-
Carbon content (wt pct)
- ∆T, ∆T c, ∆T r ∆T k :
-
Total dendritic tip undercooling, solutal undercooling, curvature undercooling, kinetics undercooling (°C)
- ∆T n, ∆T σ :
-
Average nucleation undercooling, standard deviation (°C)
- ∆r, ∆z :
-
Length and width of FD control volume (m)
- δ(∆T):
-
Increase of undercooling
- δf s :
-
Increase of solid fraction (–)
- δ c :
-
Cell size (m)
- ρ :
-
Density (kg m−3)
- Iv(P C):
-
Ivantsov function of solute Péclet number (–)
- Γ:
-
Gibbs–Thomson coefficient (–)
- λ :
-
Thermal conductivity (W m−1 K−1)
- μ :
-
Linear kinetic coefficient
- l :
-
Liquid phase
- s :
-
Solid phase
- i :
-
Solute element
- δ :
-
δ Ferrite
- γ :
-
γ Austenite
References
B. Hernandez-Morales and A. Mitchell: Ironmak. Steelmak., 1999, vol. 26, pp. 423–38.
K.M. Kelkar, S.V. Patankar, and A. Mitchell: Proceedings of 2005 International Symposium on Liquid metal Processing and Casting, Santa Fe, NM, 2005, pp. 137–44.
V. Weber, A. Jardy, B. Dussoubs, D. Ablitzer, S. Rybéron, V. Schmitt, S. Hans, and H. Poisson: Metall. Mater. Trans. B, 2009, vol. 40B, pp. 271–80.
A. Rückert and H. Pfeifer: Metal 2007, Hradec nad Moravicí, Czech Republic, 2007, pp. 1–9.
N. Giesselmann, A. Rückert and H. Pfeifer: 7th ECCC, Düsseldorf, Germany, 2011, Session 5.
Y.W. Dong, Z.H. Jiang, H. Liu, R. Chen, and Z.W. Song: ISIJ Int. 2012, vol. 52, 2226–34.
B.K. Li, B. Wang, and F. Tsukihashi: Metall. Mater. Trans. B. DOI:10.1007/s11663-013-9996-4.
S. Ahmadi, H. Arabi, A. Shokuhfar, and A. Rezaei: J. Mater. Sci. Technol., 2009, vol. 25, 592–96.
H.W. Hesselbarth, I.R. Göbel: Acta Metall. Mater., 1991, vol. 39, pp. 2135–43.
M. Rappaz and C.A. Gandin: Acta Metall. Mater., 1993, vol. 41, pp. 345–60.
C.A. Gandin and M. Rappaz: Acta Metall. Mater., 1994, vol. 42, pp. 2233–46.
M.F. Zhu and C.P. Hong: ISIJ Int., 2001, vol. 41, pp. 436–45.
M. Yamazaki, Y. Natsume, H. Harada, and K. Ohsasa: ISIJ Int., 2006, vol. 46, pp. 903–908.
S. Luo, M.Y. Zhu, and S. Louhenkilpi: ISIJ Int., 2012, vol. 52, pp. 823–30.
A. Kermanpur, D.G. Evans, R.J. Siddall, P.D. Lee, and M. Mclean: J. Mater. Sci., 2004, vol. 39, pp. 7175–82.
L. Nastac: Mater. Sci. Technol., 2012, vol. 28, pp. 1006–13.
S. Luo and M.Y. Zhu: Comput. Mater. Sci., 2013, vol. 71, pp. 10–18.
C.W. Zheng, N.M. Xiao, D.Z. Li, and Y.Y. Li: Comput. Mater. Sci., 2009, vol. 45, pp. 568–75.
M. Qian and Z.X. Guo: Mater. Sci. Eng. A, 2004, vol. 365A, pp. 180–185.
S. Kundu, M. Dutta, S. Ganguly, and S. Chandra: Scripta Mater., 2004, vol. 50, pp. 891–95.
Y.J. Lan, D.Z. Li, and Y.Y. Li: Acta Mater., 2004, vol. 52, pp. 1721–29.
X.Q. Wei and L. Zhou: Acta Metall. Sin. (Engl. Lett.), 2000, vol. 13, pp. 794–99.
J.P. Yao, L. Zhang, and H.M. Li: Foundry Technol., 2008, vol. 29, pp. 1670–73.
B.K. Li, F. Wang, H. Zhang, and M.Q. Chen: J. Iron Steel Res. Int., 2011, vol. 18, pp. 159–65.
B.K. Li, Q. Wang, F. Wang, and M.Q. Chen: JOM, 2014. DOI: 10.1007/S11837-014-0979-y.
L. Rao, X.L. Li, M.P. Geng, and Y. Zhang: Foundry, 2010, vol. 59, pp. 594–96.
L. Rao, Q.Y. Hu, X.L. Li, and M.P. Geng: Spec. Cast. Nonferr. Alloy., 2011, vol. 31, pp. 99–101.
M. Choudhary and J. Szekely: Ironmak. Steelmak., 1981, vol. 5, pp. 225–32.
W. Oldfield: ASM Trans. Quart., 1966, vol. 59, pp. 945–61.
P. Thevoz, J.L. Desbiolles, and M. Rappaz: Metall. Trans. A, 1989, vol. 20A, pp. 311–22.
M. Rappaz: Int. Mater. Rev., 1989, vol. 34, pp. 93–124.
W. Kurz, B. Giovanola, and R. Trivedi: Acta Metall., 1986, vol. 34, pp. 823–30.
W.W. Mullins and R.F. Sekerka: J. Appl. Phys., 1964, vol. 35, pp. 444–51.
M.J. Azizi: J. Appl. Phys., 1982, vol. 53, pp. 1158–68.
C.A. Gandin and M. Rappaz: Acta Mater., 1997, vol. 45, 2187–95.
S. Luo, M.Y. Zhu, C. Ji, and Z.Z. Cai: Iron Steel, 2010, vol. 45, pp. 31–36.
Y. Ueshima, S. Mizoguchi, T. Matsumiya, and H. Kajioka: Metall. Trans. B, 1986, vol. 17B, pp. 845–49.
Y.M. Won and B.G. Thomas: Metall. Mater. Trans. A, 2001, vol. 32A, pp. 1755–67.
Y. Meng, B. G. Thomas: Metall. Mater. Trans. B, 2003, vol. 34B, pp. 685–705.
K. Harste: Technical University of Clausthal, Clausthal, 1989.
Acknowledgments
This research was supported by the National High-tech Research and Development Program of China (No. 2012AA03A508, No. 2013AA030902) (863 Program) and the National Natural Science Foundation of China (No. 51274057).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted February 5, 2014.
Rights and permissions
About this article
Cite this article
Wang, X., Li, Y. Numerical Simulation of Solidification Structure of ESR Ingot Using Cellular Automaton Method. Metall Mater Trans B 46, 800–812 (2015). https://doi.org/10.1007/s11663-014-0227-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0227-4