Summary
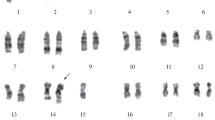
We reported that a murine carcinoma (DEN3) and its six pulmonary metastases (M2, M4C, M4D, M4E, M4F, and M6) exhibited different degrees of radioresistability (In Vitro Cell. Dev. Biol. 26:222–228; 1990). While the M2, M4C, M4E, and M4F cultured cells survived up to 2.5 Gy, the cells of DEN3 and M6 tolerated up to 5.0 Gy, and the M4D cells could withstand up to 10.0 Gy of X-irradiation. In the present investigation, the cytogenetic features of these cell lines were examined: (a) to determine the degree of cytogenetic heterogeneity among these cell lines, and (b) to investigate whether any association between the cytogenetic anomaly and the degree of radioresistability could be established. Heterogeneous cytogenetic aberrations were detected in all of the above lines. Karyotype analysis of the M4D and M6 cell lines displayed both numerical and structural abnormalities. The gain and loss of chromosomal copies were observed. Structural aberrations, such as translocation and deletion appeared in both cell lines. However, correlation between the cytogenetic abnormality and the degree of radioresistability was not demonstrated except for a dramatic reduction in one or more copies of the X-chromosome that occurred in 86% and 93% of the M6 and M4D cells, respectively. The results suggest heterogeneous cytogenetic aberrations among these cell lines and a possible association between the loss of X-chromosome and radioresistability of these tumor cells.
Similar content being viewed by others
References
Bell, C.; Frost, P.; Kerbel, R. S. Cytogenetic heterogenity of genetically marked and metastatically competent “Dominant” tumor cell clones. Cancer Genet. Cytogenet. 54:153–161; 1991.
Brison, O. Gene amplification and tumor progression. Biochim. Biophys. Acta 1155:25–41; 1993.
Calabresi, P.; Dexter, D. L. Clinical applications of cancer cell heterogeneity. In: Owens, A. H.; Coffey, D. S.; Baylin, S. B., eds. Tumor cell heterogeneity: origins and implications. New York: Academic Press; 1982:181–201.
Chakrabarti, S.; Granzow, C. Frequent involvement of Ig heavy chain carrier chromosome 12 in translocations in an Ehrlich-derived mouse tumor line. Neoplasma 39:237–239; 1992.
Cheng, K. C.; Loeb, L. A. Genomic instability and tumor progression: mechanistic considerations. Adv. Cancer Res. 60:121–156; 1993.
Committee on standardized genetic nomenclature for mice. Standard Karyotype of the mouse, Mus musculus. J. Hered. 63:69–72; 1972.
Cowell, J. K. Chromosome abnormalities associated with salivary gland epithelial cell lines transformed in vitro and in vivo with evidence of a role for genetic imbalance in transformation. Cancer Res. 41:1508–1517; 1981.
Cowell, J. K. A photographic representation of the variability in the G-banded structure of the chromosomes in the mouse karyotype. Chromosoma 89:294–320; 1984.
Dexter, D. L.; Kowalski, H. M.; Blazar, B. A., et al. Heterogeneity of tumor cells from a single mouse mammary tumor. Cancer Res. 38:3174–3181; 1978.
Hahn, P. J. Molecular biology of double minute chromosomes. BioEssays 15:477–484; 1993.
Hill, H.; Hill, J. G.; Miller, C. F., et al. Radiation and melanoma: response of B16 mouse tumor cells and clonal lines to in vitro irradiation. Radiat. Res. 80:259–276; 1979.
Hu, F.; Wang, R. Y.; Hsu, T. C. Clonal origin of metastasis in B16 murine melanoma: a cytogenetic study. JNCI 78:155–163; 1987.
Jamasbi, R. J.; Perkins, E. H. Biological heterogeneity and radiation sensitivity of in vitro propagated lung metastatic lines originated from a transplantable squamous cell carcinoma of BALB/c mouse. In Vitro Cell. Dev. Biol. 26:222–228; 1990.
Jamasbi, R. J. Generation of immunoprotection against squamous cell carcinomas by in vitro cultivation and a possible mechanism of action. Ohio J. Sci. 94:14–23; 1994.
Justice, M. J.; Siracuse, L. D.; Gilbert, D. J., et al. A genetic linkage map of mouse chromosome 10: localization of eighteen molecular markers using a single interspecific backcross. Genetics 125:855–866; 1990.
Klein, G.; Klein, E. Evolution of tumors and the impact of molecular oncology. Nature 315:190–195; 1985.
Kopelovich, L.; Chapman, T. An imbalance in sex chromosomes alters cell survival of human skin fibroblasts exposed to ionizing radiation in vitro. Cancer Genet. Cytogenet. 20:115–120; 1986.
Leith, J. T.; Dexter, D. L.; DeWyngaert, J. K., et al. Differential response to x-irradiation of sub-populations of two heterogeneous carcinomas in vitro. Cancer Res. 42:2556–2561; 1982.
Levin, N. A.; Brzoska, P.; Gupta, N., et al. Genetic alterations that correlate with radioresistance in small cell lung carcinoma. Am. Assoc. Cancer Res. Proc. 35:680–681; 1994.
Liotta, L. A.; Stetler-Stevenson, W. G. Principal of molecular cell biology of cancer: cancer metastasis. In: De Vita, V. T.; Hellman, S.; Rosenberg, S. A., eds. Cancer: principal and practice of oncology. 3rd ed. Philadelphia: J. B. Lippincott Co.; 1989:98–115.
Little, J. B. Role of P53 in radioresistance associated with expression of SV40 T-antigen. Am. Assoc. Cancer Res. Proc. 35:682; 1994.
Marx, J. L. Tumors: a mixed bag of cells. Science 215:275–277; 1982.
Nesbitt, M. N.; Francke, U. A system of nomenclature for band patterns of mouse chromosomes. Chromosoma 41:145–158; 1973.
Nguyen, H. N.; Sevin, B.-U.; Averette, H. E., et al. Evidence of tumor heterogeneity in cervical cancer and lymph node metastases as determined by flow cytometry. Cancer 71:2543–2550; 1993.
Nowell, P. C. The clonal evolution of tumor cell populations. Science 194:23–28; 1976.
Otto, E.; McCord, S.; Tisty, T. D. Increased incidence of CAD gene amplification in tumorigenic rat lines as indicator of genomic instability of neoplastic cells. J. Biol. Chem. 264:3390–3396; 1989.
Perkins, E. H.; Clapp, N. K.; Cachiro, L. H. A positive correlation between declining immune competence and early mortality associated with Diethylnitrosamine carcinogenesis in aging mice. Mech. Ageing Dev. 10:225–232; 1979.
Pierce, J. H.; Difiore, P. P.; Aaronson, S. A., et al. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a non-autocrine mechanism. Cell 41:685–693; 1985.
Potter, M.; Wiener, F. Plasmacytomagenesis in mice: model for neoplastic development upon chromosomal translocation. Carcinogenesis (Lond.) 12:1681–1697; 1992.
Runowicz, C. D.; Nuchtern, L. M.; Braunstein, J. D., et al. Heterogeneity in hormone receptor status in primary and metastatic endometrial cancer. Gynecol. Oncol. 38:437–441; 1990.
Talmadge, J. E.; Wolman, S. R.; Fidler, I. J. Evidence for the clonal origin of spontaneous metastases. Science 217:361–363; 1982.
Talmadge, J. E.; Benedict, K.; Madsen, J., et al. Development of biological diversity and susceptibility to chemotherapy in murine cancer metastases. Cancer Res. 44:3801–3805; 1984.
Trent, J. M.; Thompson, F. H. Methods for chromosome banding on human and experimental tumors in vitro. Methods Enzymol. 151:267–279; 1987.
Voncken, J. W.; Morris, C.; Patengale, P., et al. Clonal development and karyotype evolution during leukemogenesis of BCR/ABL transgenic mice. Blood 79:1029–1036; 1992.
Weichselbaum, R. R.; Dahlberg, W.; Beckett, M., et al. Radiation-resistant and repair-proficient human tumor cells may be associated with radiotherapy failure in head and neck cancer patients. Proc. Natl. Acad. Sci. 83:2684–2688; 1986.
Weichselbaum, R. R.; Beckett, M. A.; Dahlberg, W., et al. Heterogeneity of radiation response of a patient human epidermoid carcinoma cell line and four clones. Int. J. Radiat. Oncol. Biol. Phys. 14:907–912; 1988.
Weichselbaum, R. R.; Beckett, M. A.; Schwartz, J. L., et al. Radioresistant tumor cells are present in head and neck carcinomas that recur after radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 15:575–579; 1988.
Wiener, F.; Coleman, A.; Mock, B. A., et al. Nonrandom chromosomal change (trisomy 11) in murine plasmacytomas induced by an abl-myc retrovirus. Cancer Res. 55:1181–1188; 1995.
Wilson, S. D.; Billings, P. R.; D’Eustachio, P., et al. Clustering of cytokine genes on mouse chromosome 11. J. Exp. Med. 171:1301–1314; 1990.
Windle, B.; Draper, B. W.; Yin, Y., et al. A central role for chromosome breakage in gene amplification, deletion formation, and amplicon integration. Genes & Dev. 5:160–174; 1991.
Windle, B. E.; Wahl, G. M. Molecular dissection of mammalian gene amplification: new mechanistic insights revealed by analyses of very early events. Mutat. Res. 276:199–224; 1992.
Woodruff, M. F. A. Tumor clonality and its biological significance. Adv. Cancer Res. 50:197–229; 1989.
Wurster-Hill, D. H.; Cannizzaro, L. A.; Pettengill, O. S., et al. Cytogenetics of small cell carcinoma of the lung. Cancer Genet. Cytogenet. 13:303–330; 1984.
Yunis, J. J.; Sawyer, J. R.; Ball, D. W. Characterization of banding patterns of metaphase-prophase G-banded chromosomes and their use in gene mapping. Cytogenet. Cell. Genet. 22:679–683; 1978.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jamasbi, R.J., Ye, MQ. & Norvell, T.M. Cytogenetic analyses of a murine carcinoma cell line and six metastatic derivatives with different degrees of radioresistability. In Vitro Cell.Dev.Biol.-Animal 33, 137–144 (1997). https://doi.org/10.1007/s11626-997-0034-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11626-997-0034-1