Abstract
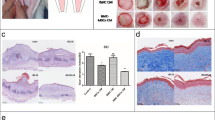
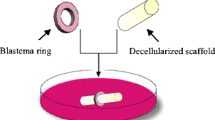
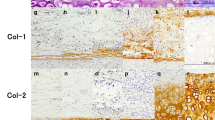
Rabbit ear wound repair is an accepted model for studies of tissue regeneration, leading to scar less wound repair. It is believed that a specific tissue, blastema, is responsible for such interesting capacity of tissue regeneration. To test this idea further and to elucidate the cellular events happening during the ear wound repair, we designed some controlled experiments in vitro. Small pieces of the ear were punched and washed immediately with normal saline. The tissues were then cultured in the Dulbecco’s Modified Eagle’s Medium, supplemented with fetal bovine serum in control group. As a treatment vitamin A and C was used to evaluate the differentiation potency of the tissue. These tissues were fixed, sectioned, stained, and microscopically studied. Micrographs of electron microscopy provided evidences revealing dedifferentiation of certain cells inside the punched tissues after incubation in tissue culture medium. The histological studies revealed that cells of the tissue (i) can undergo cellular proliferation, (ii) differentiate to epithelial, condrogenic, and osteogenic tissues, and (iii) regenerate the wounds. These results could be used for interpretation of the possible events happening during tissue engineering and wound repair in vitro. An important goal of this study is to create a tissue engineering and tissue banking model, so that in the future it could be used in further blastema tissue studies at different levels.








Similar content being viewed by others
References
Bayreuther K, Francz PI, Gogol J, Hapke C, Maier M, Meinrath HG (1991) Differentiation of primary and secondary fibroblasts in cell culture systems. Mutat Res 256:233–242
Berdanier CD (1997) Advanced nutrition micronutrients. CRC
Brockes JP, Kumar A (2002) Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol 3:566–574
Cabbabe EB, Korock SW (1986) Wound healing in vitamin C-deficient and nondeficient guinea pig: a pilot study. Ann Plast Surg 17:330–334
Combs GF (1992) The vitamins: fundamental aspects in nutrition and health. Academic, San Diego
Corcoran JP, Ferretti P (1999) RA regulation of keratin expression and myogenesis suggests different ways of regenerating muscle in adult amphibian limbs. J Cell Sci 112:1385–1394
Emura M, Mohr U, Riebe M, Aufderheide M, Dungworth DL (1988) Regulation of growth and differentiation by vitamin A in a cloned fetal lung epithelial cell line cultured on collagen gel in hormone-supplemented medium. In Vitro Cell Dev Biol 24:639–48
Fröhlich M, Malicev E, Gorensek M, Knezević M, Kregar Velikonja N (2007) Evaluation of rabbit auricular chondrocyte isolation and growth parameters in cell culture. Cell Biol Int 31:620–625
Gorensek M, Jaksimović C, Kregar-Velikonja N, Gorensek M, Knezevic M, Jeras M, Pavlovcic V, Cör A (2004) Nucleus pulposus repair with cultured autologous elastic cartilage derived chondrocytes. Cell Mol Biol Lett 9:363–373
Goss RJ (1983) Deer antlers: regeneration, function and evolution. Academic, New York
Groff JL, Gropper SS (1999) Advanced nutrition and human metabolism. West Publishing, St Paul
Hamrick I, Counts SH (2008) Vitamin and mineral supplements. Primary Care: Clin in Office Pract 35:729–747
Hay ED (1958) The fine structure of blastema cells and differentiating cartilage cells in regenerating limbs of amblystoma larvae. J Biophysic and Biochem Cytol 4:583–592
Hay ED (1959) Electron microscopic observations of muscle dedifferentiation in regenerating Amblystoma limbs. Dev Biol 1:555–585
Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79:1283–1316
Hunt TK (1986) Vitamin A and wound healing. J Am Acad Dermatol 15:817–821
Kligman LH, Duo CH, Kligman AM (1984) Topical retinoic acid enhances the repair of ultraviolet damaged dermal connective tissue. Connect Tissue Res 12:139–150
Langer R, Vacanti JP (1993) Tissue engineering. Science 260:920–926
Lanman TH, Ingalls TH (1937) Vitamin C deficiency and wound healing (experimental and clinical study). Ann Surg 105:616–625
Liosner MA, Vorontsova LD (1960) Asexual propagation and regeneration. Pergamon, New York
Mahmoudi Z, Moghaddam MM, Saeinasab M, Nakhaei-Rad S, Mirahmadi M, Mahdavi-Shahri N, Mahmoudi M, Bahrami AR (2011) Blastema cells derived from rabbit ear show stem cell characteristics. J Cell Mol Res 3:25–30
Mason JB (2007) Vitamins, trace minerals, and other micronutrients. Cecil Medicine. Saunders Elsevier, Philadelphia, chap 237
Morrison JI, Lööf S, He P, Simon A (2006) Salamander limb regeneration involves the activation of a multipotent skeletal muscle satellite cell population. J Cell Biol 172:433–440
Moskalewski S, Adamiec I, Gołaszewska A (1979) Maturation of rabbit auricular chondrocytes grown in vitro in monolayer culture. Am J Anatomy 155:339–348
Rakel D (2012) Integrative medicine, 3rd edn. Saunders Elsevier, Philadelphia
Singer AJ, Clark RAF (1999) Cutaneous wound healing. The New Eng J Med 341:738–746
Steen TP (1970) Origin and differentiative capacities of cells in the blastema of the regenerating salamander limb. Integr Comparat Biol 10:119–132
Ten Koppel PG, van Osch GJ, Verwoerd CD, Verwoerd-Verhoef HL (2001) A new in vivo model for testing cartilage grafts and biomaterials: the rabbit pinna punch-hole model. Biomaterials 22:1407–1414
Tsao CS (1997) An overview of ascorbic acid chemistry and biochemistry. Marcel Dekker, New York
Tsonis PA (2000) Regeneration in vertebrates. Dev Biol 221:273–284
Tsonis PA (2002) Regenerative biology: the emerging field of tissue repair and restoration. Differentiation 70:397–409
Tsonis PA (2004) Stem cells from differentiated cells. Mol interv 4:81–83
Uitto J (1979) Collagen polymorphism: isolation and partial characterization of alpha 1(I)-trimer molecules in normal human skin. Arch Biochem Biophys 192:371–379
Verlhac V, Gabaudan J (1994) Influence of vitamin C on the immune system of salmonids. Aquaculture Res 25:21–36
Wahli T, Verlhac V, Girling P, Gabaudan J, Aebischer C (2003) Influence of dietary vitamin C on the wound healing process in rainbow trout (Oncorhynchus mykiss). Aquaculture 225:371–386
Wallace H (1981) Vertebrate limb regeneration. Wiley, New York
Wei C, Liu X, Tao J, Wu R, Zhang P, Bian Y, Li Y, Fang F, Zhang Y (2014) Effects of vitamin C on characteristics retaining of in vitro-cultured mouse adipose-derived stem cells. In Vitro Cell Dev Biol Anim 50:75–86
Williams-Boyce PK, Daniel JC Jr (1980) Regeneration of rabbit ear tissue. J Exp Zool 212:243–253
Williams-Boyce PK, Daniel JCJR (1986) Comparison of ear tissue regeneration in mammals. J Anat 149:55–63
Yang X, Chen L, Xu X, Li C, Huang C, Deng CX (2001) TGF-β/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol 153:35–46
Acknowledgments
The authors are thankful to the Institute of Biotechnology, Ferdowsi University of Mashhad for providing work space and facilities. This project was supported by a grant from the Ferdowsi University of Mashhad.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 14 kb)
Appendix
Appendix

Rights and permissions
About this article
Cite this article
Hashemzadeh, M.R., Mahdavi-Shahri, N., Bahrami, A.R. et al. Use of an in vitro model in tissue engineering to study wound repair and differentiation of blastema tissue from rabbit pinna. In Vitro Cell.Dev.Biol.-Animal 51, 680–689 (2015). https://doi.org/10.1007/s11626-015-9868-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-015-9868-0