Abstract
Introduction
Accurate preoperative staging is important for patients with gastric cancer. This study identifies the rate of utilization of endoscopic ultrasound (EUS) and its associated factors in Medicare patients with gastric adenocarcinoma.
Methods
The linked Surveillance, Epidemiology, and End Results (SEER)-Medicare claims database was queried from 1996 to 2009 for patients with gastric cancer who underwent gastric resection. Analysis with univariate, multivariate, and Cochran-Armitage trend tests were performed.
Results
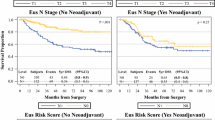
In 5826 patients with gastric cancer with an average age of 76.9 ± 6.62 years, 59.1 % had regionalized spread of cancer. EUS utilization increased significantly during the study period from 2.6 % to 22 % (p < 0.0001). EUS patients were more likely to be male, white, married, have higher education and income quartiles, and live in large metropolitan areas compared to non-EUS patients (p < 0.0001). Even after controlling for confounding factors, patients who underwent EUS were more likely to have >15 lymph nodes examined (odds ratio (OR) 1.26, 95 % confidence interval (CI) 1.04–1.53) and have the administration of both pre- and postoperative chemotherapy (OR 1.27, 95 % CI 1.03–1.57).
Conclusion
EUS is currently under-utilized but increasing. Patients who underwent EUS (12.9 %) were more likely to receive other NCCN-recommended care, including perioperative chemotherapy and adequate nodal retrieval.

Similar content being viewed by others
References
American Cancer Society and the International Agency for Research on Cancer (2011) Global Cancer Facts & Figures. 2nd edn. American Cancer Society
American Cancer Society (2014) Stomach Cancer: Detailed Guide. American Cancer Society
Stomach Cancer Factsheet (1975-2014.). SEER Cancer Statistics Review. National Cancer Institute, Bethesda, MD.
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345 (10):725-730. doi:10.1056/NEJMoa010187
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, Participants MT (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355 (1):11-20. doi:10.1056/NEJMoa055531
Fairweather M, Jajoo K, Sainani N, Bertagnolli MM, Wang J (2015) Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J Surg Oncol. doi:10.1002/jso.23919
Lei C, Huang L, Wang Y, Huang Y, Huang Y (2013) Comparison of MRI and endoscope ultrasound detection in preoperative T/N staging of gastric cancer. Mol Clin Oncol 1 (4):699-702. doi:10.3892/mco.2013.103
Mehmedovi A, Mesihovi R, Saray A, Vanis N (2014) Gastric Cancer Staging: EUS And CT. Medical Archives 68 (1):34. doi:10.5455/medarh.2014.68.34-36
Mocellin S, Pasquali S (2015) Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev 2:CD009944. doi:10.1002/14651858.CD009944.pub2
Papanikolaou IS, Triantafyllou M, Triantafyllou K, Rösch T (2011) EUS in the management of gastric cancer. Ann Gastroenterol 24 (1):9-15
Schwarz RE (2015) Current status of management of malignant disease: current management of gastric cancer. J Gastrointest Surg 19 (4):782-788. doi:10.1007/s11605-014-2707-x
Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides GA, Fields RC, Schmidt C, Weber SM, Votanopoulos K, Maithel SK, Pawlik TM (2015) Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi-institutional study of the US gastric cancer collaborative. J Am Coll Surg 220 (1):48-56. doi:10.1016/j.jamcollsurg.2014.06.023
Ajani JA, D'Amico, T.A (2015) National Comprehensive Cancer Network Gastric Cancer Guidelines, Version 3.2015. National Comprehensive Cancer Network
Gee D, Rattner, D.W. (2007) Management of Gastroesophageal Tumors. The Oncologist 12 (2):175-185. doi:10.1634/theoncologist.12-2-175
DeWitt J, Kesler K, Brooks JA, LeBlanc J, McHenry L, McGreevy K, Sherman S (2005) Endoscopic ultrasound for esophageal and gastroesophageal junction cancer: Impact of increased use of primary neoadjuvant therapy on preoperative locoregional staging accuracy. Dis Esophagus 18 (1):21-27. doi:10.1111/j.1442-2050.2005.00444.x
Hwang SW, Lee DH, Lee SH, Park YS, Hwang JH, Kim JW, Jung SH, Kim NY, Kim YH, Lee KH, Kim HH, Park do J, Lee HS, Jung HC, Song IS (2010) Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol 25 (3):512-518. doi:10.1111/j.1440-1746.2009.06106.x
Puli SR, Reddy JBK, Bechtold ML, Antillon MR, Ibdah JA (2008) How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta-analysis and systematic review. World Journal of Gastroenterology : WJG 14 (25):4011-4019. doi:10.3748/wjg.14.4011
Institute NC (2015) Healthcare Delivery Research: SEER-Medicare- Description of SEER-Medicare Database. National Institutes of Health (NIH). http://healthcaredelivery.cancer.gov/seermedicare/overview/. Accessed May 2015
Karpeh MS, Leon L, Klimstra D, Brennan MF (2000) Lymph node staging in gastric cancer: is location more important than Number? An analysis of 1,038 patients. Ann Surg 232 (3):362-371
Filik M, Kir KM, Aksel B, Soyda C, Ozkan E, Kucuk ON, Ibis E, Akgul H (2015) The Role of 18F-FDG PET/CT in the Primary Staging of Gastric Cancer. Mol Imaging Radionucl Ther 24 (1):15-20. doi:10.4274/mirt.26349
Polkowski M, Palucki J, Wronska E, Szawlowski A, Nasierowska-Guttmejer A, Butruk E (2004) Endosonography versus helical computed tomography for locoregional staging of gastric cancer. Endoscopy 36 (7):617-623. doi:10.1055/s-2004-814522
Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Winawer SJ, Urmacher C, Brennan MF (1991) Preoperative staging of gastric cancer: comparison of endoscopic US and dynamic CT. Radiology 181 (2):426-432. doi:10.1148/radiology.181.2.1924784
Ziegler K, Sanft C, Zimmer T, Zeitz M, Felsenberg D, Stein H, Germer C, Deutschmann C, Riecken EO (1993) Comparison of computed tomography, endosonography, and intraoperative assessment in TN staging of gastric carcinoma. Gut 34 (5):604-610
Ganpathi IS, So JB, Ho KY (2006) Endoscopic ultrasonography for gastric cancer: does it influence treatment? Surg Endosc 20 (4):559-562. doi:10.1007/s00464-005-0309-0
Bhandari S, Shim CS, Kim JH, Jung IS, Cho JY, Lee JS, Lee MS, Kim BS (2004) Usefulness of three-dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest Endosc 59 (6):619-626
Lee HH, Lim CH, Park JM, Cho YK, Song KY, Jeon HM, Park CH (2012) Low accuracy of endoscopic ultrasonography for detailed T staging in gastric cancer. World J Surg Oncol 10:190. doi:10.1186/1477-7819-10-190
Cardoso R, Coburn NG, Seevaratnam R, Mahar A, Helyer L, Law C, Singh S (2012) A systematic review of patient surveillance after curative gastrectomy for gastric cancer: a brief review. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 15 Suppl 1:S164-167. doi:10.1007/s10120-012-0142-9
Ishigami S, Uenosono Y, Arigami T, Yanagita S, Okumura H, Uchikado Y, Kita Y, Kurahara H, Kijima Y, Nakajo A, Maemura K, Natsugoe S (2014) Clinical utility of perioperative staging laparoscopy for advanced gastric cancer. World J Surg Oncol 12:350. doi:10.1186/1477-7819-12-350
Sarela AI, Lefkowitz R, Brennan MF, Karpeh MS (2006) Selection of patients with gastric adenocarcinoma for laparoscopic staging. Am J Surg 191 (1):134-138. doi:10.1016/j.amjsurg.2005.10.015
Nieveen van Dijkum EJ, de Wit LT, van Delden OM, Kruyt PM, van Lanschot JJ, Rauws EA, Obertop H, Gouma DJ (1999) Staging laparoscopy and laparoscopic ultrasonography in more than 400 patients with upper gastrointestinal carcinoma. J Am Coll Surg 189 (5):459-465
Reddy NK, Markowitz AB, Abbruzzese JL, Bhutani MS (2008) Knowledge of indications and utilization of EUS: a survey of oncologists in the United States. J Clin Gastroenterol 42 (8):892-896. doi:10.1097/MCG.0b013e3180cab11a
Cronin-Fenton DP, Mooney MM, Clegg LX, Harlan LC (2008) Treatment and survival in a population-based sample of patients diagnosed with gastroesophageal adenocarcinoma. World J Gastroenterol 14 (20):3165-3173
Le A, Berger D, Lau M, El-Serag HB (2007) Secular trends in the use, quality, and outcomes of gastrectomy for noncardia gastric cancer in the United States. Ann Surg Oncol 14 (9):2519-2527
Higashi T, Nakamura F, Shimada Y, Shinkai T, Muranaka T, Kamiike W, Mekata E, Kondo K, Wada Y, Sakai H, Ohtani M, Yamaguchi T, Sugiura N, Higashide S, Haga Y, Kinoshita A, Yamamoto T, Ezaki T, Hanada S, Makita F, Sobue T, Okamura T (2013) Quality of gastric cancer care in designated cancer care hospitals in Japan. Int J Qual Health Care 25 (4):418-428
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure Statement
The authors declare no conflict of interest with any persons, companies, or organizations that could bias this work or its conclusions.
Additional information
Primary Discussant
David W. Rattner, M.D. (Boston, MA)
Huntington and coauthors examined 12 years of data from the SEER and Medicare claims databases with the intent to examine the nationwide utilization of EUS and what patient or disease characteristics predicted its use. Even when controlling for confounding factors, the utilization of EUS was significantly associated with the retrieval of >15 lymph nodes at gastric resection (OR 1.26) and with pre- and postoperative chemotherapy administration – both felt to be indicator of quality in the care of gastric cancer patients. The finding that only 31 % of patients undergoing gastric resection had 15 nodes or more removed or examined should be concerning to us as members of the SSAT. My questions to you are:
1) One of the differences in care of gastric cancer patients in Korea, Japan and the USA is a different philosophy about use of neoadjuvant chemoradiation vs a good D2 lymphadenectomy. Given the relatively poor specificity of EUS for both T and N staging, is it possible that we are overtreating some patients with neoadjuvant therapy based on EUS results? Or do you think we are just covering up for an inadequate lymphadenectomy by liberal use of neoadjuvant chemoradiation?
2) EUS is highly operator dependent. Do you have any sense of quality control or improved accuracy over the course of time of your study?
3) What you have really shown in this paper is that relatively few patients with gastric cancer seem to be getting evaluated and treated in a truly multidisciplinary setting that can provide comprehensive cancer care. Given the relative rarity of gastric cancer in the USA - shouldn’t these cases viewed like pancreatic and esophageal neoplasms and be cared for at tertiary care facilities with established multidisciplinary cancer care teams?
Closing Discussant
Dr. Huntington
We would like to thank Dr. Rattner for taking the time to discuss our work and to the SSAT for the opportunity to present our research. The care of gastric cancer patients varies by geographic region, but universally, there is an increasing role for neoadjuvant therapy in locally advanced disease since the MAGIC trial and the French FNLCC/FFCD trial demonstrated an overall survival benefit for patients with preoperative chemotherapy, as well as improved success of surgical resections due to tumor downstaging. Currently, the NCCN recommends that neoadjuvant chemotherapy be considered for patients with stage II disease or any lymph node involvement (N1 or greater). Though EUS has a pooled T classification accuracy of 75 % and an 80 % pooled specificity for N classification in a meta-analysis by Dr. Cardosa and colleagues, EUS may still allow for better identification of patients who would benefit from neoadjuvant therapy prior to surgical resection. Our results actually suggest that we may be under-treating patients since less than 5 % of the patients in our study had chemotherapy prior to major gastric resection, despite the fact more than 60 % had regionalized spread of disease. Though EUS was more strongly associated with patients who had pre- and postoperative chemotherapy, these patients were also more likely to have a “good” lymphadenectomy, as defined by adequate lymph node harvest.
To your second point, our research demonstrates that the utilization of EUS has increased steadily throughout the study period. Though we cannot evaluate the variation in operator experience and interpretation through our study of this linked national dataset, a 2012 systematic review in Gastric Cancer found no significant difference in EUS accuracy in cancer staging based on the annual EUS volume.
Finally, many patients with gastric cancer in the United States could benefit from multimodal therapies to treat their disease, but our research demonstrates that the majority of older patients do not receive this. We believe that patients with gastric cancer benefit from a process of comprehensive care that includes presentation at a multidisciplinary planning conference or tumor board where medical oncologists, radiation oncologists, and surgeons can tailor individual patient care plans based on the consensus opinion of a multidisciplinary cancer care team, expert guidelines, and existing research. Community surgeons who wish to care for patients with gastric cancer should consider participating in these forums, either in person or via teleconference if this resource is not available at their facility. Referral to centers that provide comprehensive cancer care should be considered when optimal management cannot be provided at a local level.
Rights and permissions
About this article
Cite this article
Huntington, C.R., Walsh, K., Han, Y. et al. National Trends in Utilization of Endoscopic Ultrasound for Gastric Cancer: a SEER-Medicare Study. J Gastrointest Surg 20, 154–164 (2016). https://doi.org/10.1007/s11605-015-2988-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-2988-8