Abstract
Background
The receptor for advanced glycation end-products (RAGE) is a cell surface receptor implicated in tumor cell proliferation and migration. We hypothesized that RAGE signaling impacts tumorigenesis and metastatic tumor growth in murine models of colorectal carcinoma.
Materials and Methods
Tumorigenesis: Apc1638N/+ mice were crossed with Rage−/− mice in the C57BL/6 background to generate Apc1638N/+/Rage−/− mice. Metastasis: BALB/c mice underwent portal vein injection with CT26 cells (syngeneic) and received daily soluble (s)RAGE or vehicle. Rage−/− mice and Rage+/+ controls underwent portal vein injection with MC38 cells (syngeneic). Rage+/+ mice underwent portal vein injection with MC38 cells after stable transfection with full-length RAGE or mock transfection control.
Results
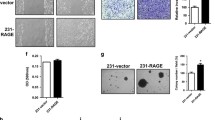
Tumorigenesis: Apc1638N/+/Rage−/− mice had reduced tumor incidence, size, and histopathologic grade. Metastasis: Pharmacological blockade of RAGE with sRAGE or genetic deletion of Rage reduced hepatic tumor incidence, nodules, and burden. Gain of function by transfection with full-length RAGE increased hepatic tumor burden compared to vector control MC38 cells.
Conclusion
RAGE signaling plays an important role in tumorigenesis and hepatic tumor growth in murine models of colorectal carcinoma. Further work is needed to target the ligand–RAGE axis for possible prophylaxis and treatment of primary and metastatic colorectal carcinoma.





Similar content being viewed by others

References
Jemal A, Siegel R., Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA cancer J Clin 2009;59:225–249.
Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D’Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575–4580.
McCart AE, Vickaryous NK, Silver A. Apc mice: Models, modifiers and mutants. Pathol Res Pract 2008:204;479–490.
Vakkila J, Lotze MT. Inflammation and necrosis promote tumor growth. Nat Rev Immunol 2004;4:641–648.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454:436–444.
Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009;457:102–107.
Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 2001;108:949–955.
Logsdon CD, Fuentes MK, Huang EH, Arumugam T. RAGE and RAGE ligands in cancer. Curr Mol Med 2007;7:777–789.
Riehl A, Németh J, Angel P, Hess J. The receptor RAGE: bridging inflammation and cancer. Cell Comm Sig 2007;7:12.
Rojas A, Figueroa H, Morales E. Fueling inflammation at tumor microenvironment: the role of multiligand/RAGE axis. Carcinogenesis 2010;31:334–341.
Ramasamy R, Yan SF, Schmidt AM. RAGE: therapeutic target and biomarker of the inflammatory response—the evidence mounts. J Leukoc Biol 2009;86:505–512.
Fuentes MK, Nigavekar SS, Arumugam T, Logsdon CD, Schmidt AM, Park JC, Huang EH. RAGE activation by S100P in colon cancer stimulates growth, migration, and cell signaling pathways. Dis Colon Rectum 2007;50:1230–1240.
Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hor O, Ogawa S, Stern DM, Schmidt AM. Blockade of amphoterin/RAGE signaling suppresses tumor growth and metastases. Nature 2001;405:354–360.
Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 2010;28:367–388.
Volp KL, Brezniceanu ML, Bosser S, Brabletz T, Kirchner T, Gottel D, Joos S, Zorniq M. Increased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon adenocarcinoma. Gut 2006;55:234–242.
Sasahira T, Akama Y, Fujii K, Kuniyasu H. Expression of receptor for advanced glycation end products and HMGB1/amphoterin in colorectal adenomas. Virchows Arch 2005;446:411–415.
Kuniyasu H, Chihara Y, Kondo H. Differential effects between amphoterin and advanced glycation end products on colon cancer cells. Int J Cancer 2003;104:722–727.
Kuniyasu H, Chihara Y, Takahashi T. Co-expression of receptor for advanced glycation end products and the ligand amphoterin associates closely with metastasis of colorectal cancer. Oncol Rep 2003;10:445–448.
Kuniyasu H, Yano S, Sasaki T, Sasahira T, Sone S, Ohmori H. Colon cancer cell-derived high mobility group 1/amphoterin induces growth inhibition and apoptosis in macrophages. Am J Pathol 2005;166:751–759.
Karin M, Greten FR. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005;5:749–759.
Karin M. Nuclear factor-κB in cancer development and progression. Nature 2006;441:431–436.
Turovskaya O, Foell D, Sinha P, Vogl T, Newlin R, Nayak J, Nguyen M, Olsson A, Nawroth PP, Bierhaus A, Varki N, Kronenberg M, Freeze HH, Srikrishna G. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis 2008;29:2035–2043.
Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Investig 1995;96:1395–1403.
Bierhaus A, Illmer T, Kasper M, Luther T, Quehenberger P, Tritschler H, Wahl P, Ziegler R, Muller M, Nawroth PP. Advanced glycation end product (AGE)-mediated induction of tissue factor in cultured endothelial cells is dependent on RAGE. Circulation 1997;96:2262–2271.
Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus. A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D’Agati VD, Schmidt AM. RAGE Drives the Development of Glomerulosclerosis and Implicates Podocyte Activation in the Pathogenesis of Diabetic Nephropathy. Am J Pathol 2003;162:1123–1137.
Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, Andrassy M, Marso SP, Duda S, Arnold B, Liliensiek B, Nawroth PP, Stern DM, Schmidt AM, Naka Y. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest 2003;111:959–972.
Harja E, Bu D, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligand in apoE −/− mice. J Clin Invest 2008;118:183–194.
Fodde R, Edelmann W, Yang K, van Leeuwen C, Carlson C, Renault B, Breukel C, Alt E, Lipkin M, Khan PM, Kucherlapati R. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci USA 1994;91:8969–8973.
Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Chow WS, Stern D. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nature Medicine 1998;4:1025–1031.
Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D, Schmidt AM. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest 1996;97:238–243.
Liang X, Romo de Vivar Chavez A, Schapiro NE, Loughran P, Thorne SH, Amoscato AA, Zeh HJ, Beer-Stolz D, Lotze MT, de Vera ME. Ethyl pyruvate administration inhibits hepatic tumor growth. J Leukoc Biol 2009;86:599–607
Kostova N, Zlateva S, Ugrinova I, Pasheva E. The expression of HMGB1 protein and its receptor RAGE in human malignant tumors. Mol Cell Biochem 2010;337:251–258.
Acknowledgments
This work was generously supported by the I.W. Foundation and an institutional Ruth L. Kirschstein National Research Service Award (T32 HL 007854-14).
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. Emina H. Huang (Gainesville, FL): This is the lab where I started as faculty, and it’s really nice to see you guys progress with your work. So I would like to congratulate you for continuing this investigation of RAGE and colorectal cancer.
As you point out, RAGE activation has been implicated in a broad range of disease processes, including inflammation and diabetes as well as cancer. In the current studies, your team uses two different murine models.
In the first model, you have spontaneous development of adenomas and adenocarcinoma. However, this model bears the shortcomings of many murine polyposis-like models, in which tumors are dominantly present in the small bowel rather than in the colon.
In your second model, you directly inject the portal vein, which results in the development of liver lesions. Despite shortcomings associated with any model system, the significance of your studies reveals the potential for future translation. So I have a couple of questions:
Number one, would you posit that an orthotopic metastatic model of colorectal cancer might demonstrate different results? And number two, chronic inflammation is involved in 15% of the world’s malignancies. Certainly, these relationships are seen in the GI tract, including Barrett’s esophagus, hepatitis, and ulcerative colitis. Would you envision RAGE antagonism as having a role in chemo prevention or in an adjuvant cancer treatment?
Thank you. Keep up the great work.
Closing Discussant
Dr. Joseph DiNorcia: Thank you, Dr. Huang, for your gracious comments and thoughtful questions. To answer the first, it’s true that the majority of tumors we saw in the Apc 1638 model developed in the small intestine, as is reported in literature. Though of small intestine origin, these tumors follow the same adenoma to carcinoma progression as a colon lesion. So I hope an orthotopic model would demonstrate similar effects. A colleague at the resident research conference suggested using a chemical-induced model of colon carcinogenesis to test the effects of RAGE. Using AOM/DSS, for example, we might induce colon cancer in both RAGE knockout and RAGE wild-type mice and compare results.
To answer the second question, I think the ligand–RAGE axis is an exciting potential target for both prevention and treatment of cancer. In terms of treatment, it’s known that when they outgrow their blood supply, tumor cells necrose and release HMGB1. HMGB1 then feeds back on RAGE to create a pro-survival environment that supports the remaining tumor cells. So it’s very possible that a RAGE antagonist could inhibit that feedback mechanism and act either as a primary therapy or as an adjunct agent that might make chemotherapy even more effective. Still, we have a lot more work to do before we get to the point of using a RAGE antagonist in clinical practice.
Discussant
Dr. Merril Dayton (Buffalo, NY): A group at my home institution in Buffalo is studying RAGE in trauma and has found that animals that have a high RAGE diet do much more poorly after trauma. My question for you is, what are the implications of diet on advanced glycosylation end products and so forth? Have you had any opportunity to pre-feed animals RAGE products and see how that impacts cancer? Obviously, there is concern about carbonized food products and its implications in colon cancer.
Closing Discussant
Dr. Joseph DiNorcia: We haven’t studied any of the potential dietary effects in these mouse models of cancer. It’s true that most of the original work on RAGE was done in diabetes. And certainly in the tumor microenvironment, there are increased levels of advanced glycosylation end products, as tumor cells have increased rates of glycolysis. It follows then that diets high in refined carbohydrates might predispose to the development of cancer, perhaps mediated through the ligand–RAGE axis. It would be an interesting area for future study.
Rights and permissions
About this article
Cite this article
DiNorcia, J., Moroziewicz, D.N., Ippagunta, N. et al. RAGE Signaling Significantly Impacts Tumorigenesis and Hepatic Tumor Growth in Murine Models of Colorectal Carcinoma. J Gastrointest Surg 14, 1680–1690 (2010). https://doi.org/10.1007/s11605-010-1347-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-010-1347-z