Abstract
Introduction
Pancreatic cancer is one of the leading causes of cancer-related death in the USA. Recently, several centers have introduced portal and superior mesenteric vein resection and reconstruction during extended pancreatectomy, rendering the previously inoperable cases resectable.
Aim
The aim of this study is to confirm whether patients with locally advanced pancreatic cancer and mesenteric vascular invasion can be cured with extended pancreatectomy with vascular reconstruction (VR) and to compare their survival to patients treated with pancreatectomy without VR and those treated without resection (palliation).
Methods
Survival of 22 patients who underwent pancreatectomy with VR was compared with two control groups: 54 patients who underwent pancreatectomy without the need for VR and 28 patients whose pre-operative imaging suggested resectability but whose laparotomy indicated inoperability.
Results
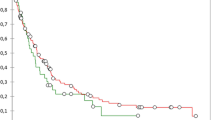
A slight survival benefit was noted in patients who did not require VR (33.5%) compared to those who did require VR [20%, p = 0.18], although not reaching statistical significance. Despite a low 15% three-year survival in patients treated palliatively, this was not statistically different compared to survival after resection with VR (P = 0.23). The presence of nodal metastasis was associated with worse survival (p = 0.006), and the use of adjuvant therapy was associated with better survival (p = 0.001).
Conclusion
Pancreatic cancers that require VR to completely resect the tumor have a similar survival to those not requiring VR. Long-term survival was achievable in approximately 1 out 5 patients requiring VR, although we were not able to demonstrate statistically improved survival compared to palliative care.

Similar content being viewed by others
References
Jemal A, Siegel R, Ward E et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66.
Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg 2003;237:74–85.
Cameron JL, Crist DW, Sitzmann JV, Hruban RH, Boitnott JK, Seidler AJ, Coleman J. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg 1991;161:120–124 (discussion 124–125).
Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567–579.
Fleming ID, American Joint Committee on Cancer, American Cancer Society, American College of Surgeons. AJCC Cancer Staging Manual. Hagerstown, MD: Lippincott-Raven, 1997.
Greene FL, American Joint Committee on Cancer, American Cancer Society. AJCC Cancer Staging Manual, 6th ed. New York: Springer-Verlag, 2002.
Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, Gomez HF, Sun CC, Crane CH, Wolff RA, Evans DB. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg 2004;8:935–949 (discussion 949–950).
Tempero MA, Behrman S, Ben-Josef E et al. Pancreatic adenocarcinoma. Clinical Practice Guidelines in Oncology. J Natl Comprehensive Cancer Network 2005;3(5):598–626.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–655.
Moore GE, Sako Y, Thomas LB. Radical pancreatoduodenectomy with resection and reanastomosis of the superior mesenteric vein. Surgery 1951;30:550–553.
Symbas PN, Foster JH, Scott HW Jr. Experimental vein grafting in the portal venous system. Surgery 1961;50:97–106.
Asada S, Itaya H, Nakamura K, Isohashi T, Masuoka S. Radical Pancreatoduodenectomy and portal vein resection. Report of two successful cases with transplantation of portal vein. Arch Surg 1963;87:609–613.
Fortner JG. Regional resection of cancer of the pancreas: a new surgical approach. Surgery 1973;73:307-320.
Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 2002;236:355–366 (discussion 366–368).
Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Gines MA, Real MI, Gilabert R, Quinto L, Trilla A, Feu F, Montanya X, Fernandez-Cruz L, Navarro S. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol 2004;99:492–501.
Varadhachary GR, Tamm EP, Abruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of perioperative therapy. Ann Surg Oncol 2006;13:1035–1046.
Martin JK, Goellner JR. Abdominal fluid cytology in patients with gastrointestinal malignant lesions. Mayo Clin Proc 1986;61:467–471.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Haddad, M., Martin, J.K., Nguyen, J. et al. Vascular Resection and Reconstruction for Pancreatic Malignancy: A Single Center Survival Study. J Gastrointest Surg 11, 1168–1174 (2007). https://doi.org/10.1007/s11605-007-0216-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-007-0216-x