Summary
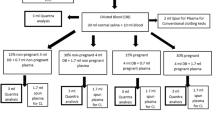
Sonoclot analyzer has been widely used in many countries. But the reference intervals provided by the manufacturer were derived from only 45 participants, and there was no cut-off value for transfusion for Sonoclot analysis. This study aimed to establish reference intervals and transfusion criterion for Sonoclot analysis. Volunteers were recruited from healthy Chinese adults and patients undergoing cardiac surgery. Blood samples were withdrawn from forearm vein and measured for activated clotting time (ACT), clot rate (CR), platelet function (PF), activated partial thromboplastin time (APTT), fibrinogen concentration (FIB), and platelet count (PLT). The reference intervals were determined by the nonparametric method. Cut-off values were determined by the receiver operating characteristics curve. A total of 135 healthy volunteers and 281 patients were enrolled. The 95% reference intervals were 96–195 s, 22–51 signal U/min, >1.6 for ACT, CR, PF respectively. In the 281 patients, the results of APTT, FIB, PLT, ACT, CR, and PF ranged from 20.5–300.0 s, 0.28–4.11 g/L, (19.0–387.3)×109/L, 80–514 s, 2.9–74 signal U/min, and 0.1–5.1 respectively. The cut-off values for transfusion were >208, ≤14, and ≤1.3 for ACT, CR, PF respectively. The cut-off values of Sonoclot analysis were within the manufacturer’s reference intervals, while they were outside the reference intervals established in this study. The results suggested that the manufacturer’s reference intervals were not suitable for Chinese. The reference intervals and cut-off values established in this study will be helpful to Chinese patients.
Similar content being viewed by others
References
Lee B, AI-Waili N, Butler G, et al. Assessment of heparin anticoagulation by Sonoclot Analyzer in arterial reconstruction surgery. Technol Health Care, 2011,19(19):109–114
Schott U, Nilsson LG, Broman M, et al. Monitoring of low molecular weight heparin anticoagulation during haemodialysis with a Sonoclot Analyzer. Perfusion, 2010,25(4):191–196
Miyashita T, Kuro M. Evaluation of platelet function by sonoclot analysis compared with other hemostatic variables in cardiac surgery. Anesth Analg, 1998,87(6):1228–1233
Chapin JW, Becker GL, Hulbert BJ, et al. Comparison of thromboelastograph and Sonoclot coagulation analyzer for assessing coagulation status during orthotopic liver transplantation. Transplant Proc, 1989,21(3):3539
Bindi ML, Biancofiore GD, Consani G, et al. Blood coagulation monitoring during liver transplantation: Sonoclot analysis and laboratory tests. Minerva Anestesiol, 2001,67(5):359–369
Sieders E, De Somer F, Bouchez S, et al. Haemostasis monitoring during sequential aortic valve replacement and liver transplantation. Acta Gastroenterol Belg, 2010,73(1): 65–68
Nuttall GA, Oliver WC, Ereth MH, et al. Coagulation tests predict bleeding after cardiopulmonary bypass. J Cardiothorac Vasc Anesth, 1997,11(7):815–823
Yamada T, Katori N, Tanaka KA, et al. Impact of Sonoclot hemostasis analysis after cardiopulmonary bypass on postoperative hemorrhage in cardiac surgery. J Anesth, 2007,21(2):148–152
Sienco, Inc., Product Inserts. Available at: http://www.sienco. com/sonoclot-worldwide /product-inserts.html. Accessed March 30, 2013
Padhi S, Kemmis-Betty S, Rajesh S, et al. Blood transfusion: summary of NICE guidance. BMJ, 2015,351(2):395–396
Clinical and Laboratory Standards Institute. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory, Approved Guideline. Third Edition, CLSI document C28-A3. Wayne: Clinical and Laboratory Standards Institute, 2008
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Zl., Chen, Yp., Tao, Ch. et al. Establishment of reference intervals and transfusion criterion for Sonoclot analysis. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 36, 614–617 (2016). https://doi.org/10.1007/s11596-016-1634-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-016-1634-3