Summary
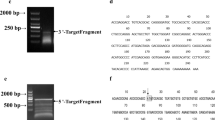
In order to confirm the existence of indoleamine 2, 3-dioxygenase (IDO) gene in swine, and to clone the novel gene followed by the molecule structure properties and expression pattern analysis, the porcine mRNA sequences homologous to human IDO were obtained from GenBank database by bioinformatics method. By using RT-PCR, the IDO gene was cloned from porcine endothelial cell line and the accuracy of the nucleic acid sequence was confirmed, and the expression pattern of the gene was detected. The three-dimensional structure model of porcine IDO was built referring to the tertiary structure of human IDO using biological sequence analysis software and database. The results showed that the porcine IDO was identified by sequencing. The nucleotide sequences were confirmed as a novel gene after submitted to Genbank. Porcine IDO was expressed in the lung, thymus, epididymis and anterior chamber with a basic level, however in peripheral blood mononuclear cells (PBMCs) the IDO gene was highly expressed. The three-dimensional structure model of porcine IDO was similar to that of human IDO. It was suggested that identification of the structure information of porcine IDO is essential to further investigate the immunologic function of the gene. Study of IDO on NK cells-mediated xenograft rejection will be a novel therapeutic target for the development of xenotransplantation.
Similar content being viewed by others
References
Peters JC. Tryptophan nutrition and metabolism: an overview. Adv Exp Med Biol, 1991,294:345–358
Hayaishi O. My life with tryptophan-never a dull moment. Protein Sci, 1993,2(3):472–475
Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol, 2004,4(10):762–774
Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science, 1998,281(5380):1191–1193
Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2, 3-dioxygenase. Nat Med, 2003,9(10):1269–1274
Curti A, Aluigi M, Pandolfi S, et al. Acute myeloid leukemia cells constitutively express the immunoregulatory enzyme indoleamine 2, 3-dioxygenase. Leukemia, 2007,21(2):353–355
Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signaling and non-canonical NF-kappa B activation. Nat Rev Immunol, 2007,7(10):817–823
Grohmann U, Fallarino F, Puccetti P, et al. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol, 2003,24(5):242–248
Valdes-Gonzalez R, Silva-Torres L, Ramirez-Gonzalez B, et al. Method for evaluating quality of cultured neonatal pig Sertoli cells. Xenotransplantation, 2005,12(4):316–323
Kozak M. A consideration of alternative models for the initiation of translation in eukaryotes. Crit Rev Biochem Mol Biol, 1992,27(4–5):385–402
Hirata F, Ohnishi T, Hayaishi O. Indoleamine 2, 3-dioxygenase. Characterization and properties of enzyme. O2- complex. J Biol Chem, 1977,252(13):4637–4642
Littlejohn TK, Takikawa O, Truscott RJ, et al. Asp274 and his346 are essential for heme binding and catalytic function of human indoleamine 2, 3-dioxygenase. J Biol Chem, 2003,278(32):29 525–29 531
Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol, 2003,81(4):247–265
Sachs DH. The pig as a xenograft donor. Vet Immunol Immunopathol, 1994,43(1–3):217–219
Seebach JD, Waneck GL. Natural killer cells in xenotransplantation. Xenotransplantation, 1997,4(4):201–211
Goodman DJ, Von Albertini M, Willson A, et al. Direct activation of porcine endothelial cells by human natural killer cells. Transplantation, 1996,61(5):763–771
Frumento G, Rotondo R, Tonetti M, et al. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2, 3-dioxygenase. J Exp Med, 2002,196(4): 459–468
Miki T, Sun H, Lee Y, et al. Blockade of tryptophan catabolism prevents spontaneous tolerogenicity of liver allografts. Transplant Proc, 2001,33(1–2):129–130
Beutelspacher SC, Pillai R, Watson MP, et al. Function of indoleamine 2, 3-dioxygenase in corneal allograft rejection and prolongation of allograft survival by over-exp ression. Eur J Immunol, 2006,36(3):690–700
Logan GJ, Smyth CM, Earl JW, et al. HeLa cells cocultured with peripheral blood lymphocytes acquire an immunoinhibitory phenotype through up-regulation of indoleamine 2, 3-dioxygenase activity. Immunology, 2002,105(4):478–487
Lin YC, Chen CL, Nakanao T, et al. Immunological role of indoleamine 2, 3-dioxygenase in rat liver allograft rejection and tolerance. J Gastroenterol Hepatol, 2008,23(7 Pt 2):e243–250
Guillonneau C, Hill M, Hubert FX, et al. CD40Ig treat ment results in allograft acceptance mediated by CD8CD45RC T cells, IFN-gamma, and indoleamine 2, 3-dioxygenase. J Clin Invest, 2007,117(4):1096–1106
Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol, 1999,17:875–904
Moretta, Bottino C, Pende D, et al. Different checkpoints in human NK-cell activation. Trends Immunol, 2004,25(12):670–676
Vitale M, Bottino C, Sivori S, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells is involved in non-MHC restricted tumor cell lysis. J Exp Med, 1998,187(12):2065–2072
Moretta A, Poggi A, Pende D, et al. CD69-mediated pathway of lymphocyte activation: anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor alpha/beta. J Exp Med, 1991,174(6):1393–1398
Forte P, Lilienfeld BG, Baumann BC, et al. Human NK cytotoxicity against porcine cells is triggered by NKp44 and NKG2D. J Immunol, 2005,175(8):5463–5470
Della Chiesa M, Carlomagno S, Frumento G, et al. The tryptophan catabolite L-kynuren ine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood, 2006,108(13): 4118–4125
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by grants from Wuhan Youth Chenguang Project (No. 201050231077) and National Natural Science Foundation of China (No. 30600571 and No. 81172786).
Rights and permissions
About this article
Cite this article
Chen, C., Wei, M., Wang, L. et al. Molecular cloning and characterization of porcine indoleamine 2, 3-dioxygenase and its expression in various tissues. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 32, 473–479 (2012). https://doi.org/10.1007/s11596-012-0082-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-012-0082-y