Abstract
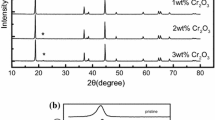
LiNi0.5Mn1.5O4 (LNMO) is a promising cathode material for lithium-ion batteries due to its high discharge voltage, low cost, environmental friendliness, and high energy density. To enhance the electrochemical performance of LNMO, Cd is innovatively employed as a modifying agent. Structural and morphological characterizations confirm that CdO is successfully wrapped on the surface of LNMO. Further physical, chemical, and electrochemical detections verify the phase transition of LNMO. The CdO-coated samples show excellent cyclability and rate performance. The CdO-coated LNMO (Cd 0.4 wt.%) exhibits a discharge capacity of 133.3 mAh g−1 with a retention rate of 95.2% after 300 cycles at 1-C rate (3.5–4.8 V), while that of the pristine is only 89.4%. Even at 10-C rates, the CdO-coated LNMO has an initial discharge capacity of 120.0 mAh g−1 with a retention rate of 92.3% after 300 cycles at the same voltage limit. Specifically, at the first of cycling, the newly formed Cd-F compounds and CdO layer could suppress the side reactions and reduce the formation of surface film. Finally, the process of kinetics is improved.









Similar content being viewed by others
References
Kim JW, Kim DH, Oh DY, Lee H, Kim JH, Lee JH, Jung YS (2015) Surface chemistry of LiNi0.5Mn1.5O4 particles coated by Al2O3using atomic layer deposition for lithium-ion batteries. J Power Sources 274:1254–1262. https://doi.org/10.1016/j.jpowsour.2014.10.207
Liu J, Cheng Y, Fan Q, Zhang L, Liu L, Ke X, Wang N, Shi Z, Guo Z (2018) Tri-functional coating to enhance the capacity retention of LiNi0.5Mn1.5O4 for high power lithium ion battery. Mater Lett 214:68–71. https://doi.org/10.1016/j.matlet.2017.11.046
Amin R, Belharouak I (2017) Part-II: exchange current density and ionic diffusivity studies on the ordered and disordered spinel LiNi0.5Mn1.5O4 cathode. J Power Sources 348:318–325. https://doi.org/10.1016/j.jpowsour.2017.02.070
Shi K, Liang K, Zheng J, Qiu Y (2019) Enhanced electrochemical performance of Li3V1.5Al0.5(PO4)3-modified Li1.12(Ni0.18Co0.07Mn0.57)O2 cathode for Li-ion batteries. Appl Surf Sci 483:1–9. https://doi.org/10.1016/j.apsusc.2019.03.115
Yi TF, Han X, Chen B, Zhu YR, Xie Y (2017) Porous sphere-like LiNi0.5Mn1.5O4-CeO2composite with high cycling stability as cathode material for lithium-ion battery. J Alloys Compd 703:103–113. https://doi.org/10.1016/j.jallcom.2017.01.342
Lu BH, Zhou J, Li YY, Xiao W, Wang HL (2015) Comparison on carbon coating over silica spheres with different carbon sources. Mater Technol 30:A94–A98. https://doi.org/10.1179/17535557A15Y.000000009
Choi DI, Lee H, Lee DJ, Nam KW, Kim JS, Huggins RA, Park JK, Choi JW (2013) Cotton-templated hierarchical porous structures for high power lithium rechargeable batteries. J Mater Chem A 1:5320. https://doi.org/10.1039/c3ta00192j
Santhanam R, Rambabu B (2010) Research progress in high voltage spinel LiNi0.5Mn1.5O4 material. J Power Sources 195:5442–5451. https://doi.org/10.1016/j.jpowsour.2010.03.067
Wang H, Xie X, Wei X, Zhang X, Zhang J, Huang Y, Li Q (2017) A new strategy to stabilize capacity and insight into the interface behavior in electrochemical reaction of LiNi0.5Mn1.5O4/graphite system for high-voltage lithium-ion batteries. ACS Appl Mater Interfaces 9:33274–33287. https://doi.org/10.1021/acsami.7b08828
Yoon T, Park S, Mun J, Ryu JH, Choi W, Kang YS, Park JH, Oh SM (2012) Failure mechanisms of LiNi0.5Mn1.5O4 electrode at elevated temperature. J Power Sources 215:312–316. https://doi.org/10.1016/j.jpowsour.2012.04.103
Xue Y, Han Y, Wang Z et al (2018) Cathode for lithium-ion batteries. 4:3317–3323
Samarasingha PB, Sottmann J, Margadonna S, Emerich H, Nilsen O, Fjellvåg H (2016) In situ synchrotron study of ordered and disordered LiNi0.5Mn1.5O4 as lithium ion battery positive electrode. Acta Mater 116:290–297. https://doi.org/10.1016/j.actamat.2016.06.040
Xue Y, Han Y, Yu H et al (2018) Electrochimica Acta Improving rate performance of high-voltage spinel cathode by changing structural evolution from two-phase to solid-solution reactions. Electrochim Acta 281:24–30. https://doi.org/10.1016/j.electacta.2018.05.146
Yi TF, Mei J, Zhu YR (2016) Key strategies for enhancing the cycling stability and rate capacity of LiNi0.5Mn1.5O4 as high-voltage cathode materials for high power lithium-ion batteries. J Power Sources 316:85–105. https://doi.org/10.1016/j.jpowsour.2016.03.070
Atanasov M, Barras J-L, Benco L, Daul C (2000) Electronic structure, chemical bonding, and vibronic coupling in Mn IV /Mn III mixed valent LixMn2O4 spinels and their effect on the dynamics of intercalated Li: a cluster study using DFT. J Am Chem Soc 122:4718–4728. https://doi.org/10.1021/ja9904484
Haridas AK, Sharma CS, Rao TN (2016) Caterpillar-like sub-micron LiNi0.5Mn1.5O4 structures with site disorder and excess Mn3+ as high performance cathode material for lithium ion batteries. Electrochim Acta 212:500–509. https://doi.org/10.1016/j.electacta.2016.07.039
Mou J, Deng Y, He L et al (2018) Critical roles of semi-conductive LaFeO3 coating in enhancing cycling stability and rate capability of 5 V LiNi0.5Mn1.5O4 cathode materials. Electrochimica Acta 260:101–111. https://doi.org/10.1016/j.electacta.2017.11.059
Fan Y, Wang J, Tang Z et al (2007) Effects of the nanostructured SiO2 coating on the performance of LiNi0.5Mn1.5O4 cathode materials for high-voltage Li-ion batteries. 52:3870–3875. https://doi.org/10.1016/j.electacta.2006.10.063
Won Bin Ima,, Byung Won Choa, Hyung-Sun Kima, Hee Lack Choib, Hae Yong Jungb, Kyoon C (2009) A study on the ZrO2 coating effect on the electrochemical performance of LiNi0.5Mn1.5O4 won. Electrochem Soc A 2018
Wu HM, Belharouak I, Abouimrane A et al (2010) batteries 195:2909–2913. https://doi.org/10.1016/j.jpowsour.2009.11.029
Konishi H, Suzuki K, Taminato S, Kim K, Zheng Y, Kim S, Lim J, Hirayama M, Son JY, Cui Y, Kanno R (2014) Effect of surface Li3PO4 coating on LiNi0.5Mn1.5O4 epitaxial thin fi lm electrodes synthesized by pulsed laser deposition. J Power Sources 269:293–298. https://doi.org/10.1016/j.jpowsour.2014.05.052
Hee W, Chul M, Nip S et al (2014) Enhanced elevated temperature performance of LiFePO4 modified spinel LiNi0.5Mn1.5O4 cathode. 612:51–55. https://doi.org/10.1016/j.jallcom.2014.05.149
Xu Y, Zhao S, Deng Y et al (2016) Improved electrochemical performance of 5 V spinel LiNi0.5Mn1.5O4 microspheres by F-doping and Li4SiO4 coating. 2:265–272. https://doi.org/10.1016/j.jmat.2016.04.005
Gupta RK, Ghosh K, Patel R et al (2008) Structural, optical and electrical properties of In doped CdO thin fi lms for optoelectronic applications. 62:3373–3375. https://doi.org/10.1016/j.matlet.2008.03.015
Feng J, Xiong S, Qian Y, Yin L (2014) Electrochimica Acta Synthesis of nanosized cadmium oxide ( CdO ) as a novel high capacity anode material for Lithium-ion batteries : influence of carbon nanotubes decoration and binder choice. Electrochim Acta 129:107–112. https://doi.org/10.1016/j.electacta.2014.02.085
Guerrero Moreno R, Takeuchi N (2002) First principles calculations of the ground-state properties and structural phase transformation in CdO
Hong D, Guo Y, Wang H, Zhou J, Fang HT (2015) Mechanism for improving the cycle performance of LiNi0.5Mn1.5O4 by RuO2 surface modification and increasing discharge cut-off potentials. J Mater Chem A 3:15457–15465. https://doi.org/10.1039/c5ta02255j
Cho JH, Park JH, Lee MH, Song HK, Lee SY (2012) A polymer electrolyte-skinned active material strategy toward high-voltage lithium ion batteries: a polyimide-coated LiNi0.5Mn1.5O4 spinel cathode material case. Energy Environ Sci 5:7124–7131. https://doi.org/10.1039/c2ee03389e
Mou J, Deng Y, He L, Zheng Q, Jiang N, Lin D (2018) Critical roles of semi-conductive LaFeO3coating in enhancing cycling stability and rate capability of 5 V LiNi0.5Mn1.5O4 cathode materials. Electrochim Acta 260:101–111. https://doi.org/10.1016/j.electacta.2017.11.059
Li X, Guo W, Liu Y, He W, Xiao Z (2014) Spinel LiNi0.5Mn1.5O4 as superior electrode materials for lithium-ion batteries: ionic liquid assisted synthesis and the effect of CuO coating. Electrochim Acta 116:278–283. https://doi.org/10.1016/j.electacta.2013.11.055
Deng Y, Mou J, Wu H, Jiang N, Zheng Q, Lam KH, Xu C, Lin D (2017) A superior Li2SiO3-composited LiNi0.5Mn1.5O4 cathode for high-voltage and high-performance lithium-ion batteries. Electrochim Acta 235:19–31. https://doi.org/10.1016/j.electacta.2017.03.066
Yin C, Zhou H, Yang Z, Li J (2018) Synthesis and electrochemical properties of LiNi0.5Mn1.5O4 for Li–ion batteries by the metal–organic framework method. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.8b02553
Zhang X, Cheng F, Zhang K, Liang Y, Yang S, Liang J, Chen J (2012) Facile polymer-assisted synthesis of LiNi0.5Mn1.5O4 with a hierarchical micro–nano structure and high rate capability. RSC Adv 2:5669. https://doi.org/10.1039/c2ra20669b
Li J, Wang H, Dong W, Shi Z, Xie W, Qiao H, Yu Q, Zhang M, Hu J, Yang L, Hong J (2018) Phase transition dominated high-rate performances of the high voltage LiNi0.5Mn1.5O4 cathode: improvement on structure evolution and ionic diffusivity by chromium doping. J Phys Chem C 122:25229–25236. https://doi.org/10.1021/acs.jpcc.8b09054
Deng Y-F, Zhao S-X, Zhai P-Y, Cao G, Nan CW (2015) Impact of lithium excess on the structural and electrochemical properties of the LiNi0.5Mn1.5O4 high-voltage cathode material. J Mater Chem A 3:20103–20107. https://doi.org/10.1039/C5TA06339F
Lee K, Yang GJ, Kim H, Kim T, Lee SS, Choi SY, Choi S, Kim Y (2017) Composite coating of Li2O–2B2O3 and carbon as multi-conductive electron/Li-ion channel on the surface of LiNi0.5Mn1.5O4 cathode. J Power Sources 365:249–256. https://doi.org/10.1016/j.jpowsour.2017.08.080
Yi TF, Li YM, Li XY, Pan JJ, Zhang Q, Zhu YR (2017) Enhanced electrochemical property of FePO4-coated LiNi0.5Mn1.5O4 as cathode materials for Li-ion battery. Sci Bull 62:1004–1010. https://doi.org/10.1016/j.scib.2017.07.003
Ma F, Geng F, Yuan A, Xu J (2017) Facile synthesis and characterization of a SnO2 -modified LiNi0.5Mn1.5O4 high-voltage cathode material with superior electrochemical performance for lithium ion batteries. Phys Chem Chem Phys 19:9983–9991. https://doi.org/10.1039/C7CP00943G
Wang Y, Peng Q, Yang G, Yang Z, Zhang L, Long H, Huang Y, Lu P (2014) High-stability 5 v spinel LiNi0.5Mn1.5O4 sputtered thin film electrodes by modifying with aluminium oxide. Electrochim Acta 136:450–456. https://doi.org/10.1016/j.electacta.2014.04.184
Gao X-W, Deng Y-F, Wexler D, Chen GH, Chou SL, Liu HK, Shi ZC, Wang JZ (2015) Improving the electrochemical performance of the LiNi0.5Mn1.5O4 spinel by polypyrrole coating as a cathode material for the lithium-ion battery. J Mater Chem A 3:404–411. https://doi.org/10.1039/C4TA04018J
Sun P, Ma Y, Zhai T, Li H (2016) High performance LiNi0.5Mn1.5O4cathode by Al-coating and Al3+-doping through a physical vapor deposition method. Electrochim Acta 191:237–246. https://doi.org/10.1016/j.electacta.2016.01.087
Nisar U, Amin R, Essehli R et al (2018) Extreme fast charging characteristics of zirconia modified LiNi0.5Mn1.5O4 cathode for lithium ion batteries. 396:774–781. https://doi.org/10.1016/j.jpowsour.2018.06.065
Lu D, Xu M, Zhou L, Garsuch A, Lucht BL (2013) Failure mechanism of graphite/ LiNi0.5Mn1.5O4 cells at high voltage and elevated temperature. J Electrochem Soc 160:A3138–A3143. https://doi.org/10.1149/2.022305jes
Mansour AN, Kwabi DG, Quinlan RA, Lu YC, Shao-Horn Y (2016) Probing the electrode-electrolyte interface in cycled LiNi0.5Mn1.5O4 by XPS using mg and synchrotron X-rays. J Electrochem Soc 163:A2911–A2918. https://doi.org/10.1149/2.0331614jes
Aktekin B, Lacey MJ, Nordh T, Younesi R, Tengstedt C, Zipprich W, Brandell D, Edström K (2018) Understanding the capacity loss in LiNi0.5Mn1.5O4-Li4Ti5O12 Lithium-ion cells at ambient and elevated temperatures. J Phys Chem C 122:11234–11248. https://doi.org/10.1021/acs.jpcc.8b02204
Briggs D, Beamson G (1992) Primary and secondary oxygen-induced C1s binding energy shifts in X-ray photoelectron spectroscopy of polymers. Anal Chem 64:1729–1736. https://doi.org/10.1021/ac00039a018
Sang M, Sik D, Park E et al (2016) Simultaneous fluorination of active material and conductive agent for improving the electrochemical performance of LiNi0.5Mn1.5O4 electrode for lithium-ion batteries. J Power Sources 326:156–161. https://doi.org/10.1016/j.jpowsour.2016.06.130
Cho H, Chen MV, Macrae AC, Meng YS (2015) Hyung-Man Cho, Michael Vincent Chen, Alex C. MacRae, and Ying Shirley Meng . https://doi.org/10.1021/acsami.5b01392, Effect of surface modification on nano-structured LiNi0.5Mn1.5O4 spinel materials
Talyosef Y, Markovsky B, Salitra G et al (2005) The study of LiNi0.5Mn1.5O4 5-V cathodes for Li-ion batteries. 146:664–669. https://doi.org/10.1016/j.jpowsour.2005.03.064
Patoux S, Daniel L, Bourbon C et al (2009) High voltage spinel oxides for Li-ion batteries: from the material research to the application. 189:344–352. https://doi.org/10.1016/j.jpowsour.2008.08.043
Yi T-F, Chen B, Zhu Y-R, Li X-Y, Zhu R-S (2014) Author’s personal copy enhanced rate performance of molybdenum-doped spinel. J Power Sources 247:778–785
Wang H, Shi Z, Li J, Yang S, Ren R, Cui J, Xiao J, Zhang B (2015) Direct carbon coating at high temperature on LiNi0.5Mn1.5O4 cathode: unexpected influence on crystal structure and electrochemical performances. J Power Sources 288:206–213. https://doi.org/10.1016/j.jpowsour.2015.04.137
Li J, Li S, Xu S, Huang S, Zhu J (2017) Synthesis and electrochemical properties of LiNi0.5Mn1.5O4 cathode materials with Cr3+ and F− composite doping for lithium-ion batteries. Nanoscale Res Lett 12(414):414. https://doi.org/10.1186/s11671-017-2172-z
Feng S, Kong X, Sun H, Wang B, Luo T, Liu G (2018) Effect of Zr doping on LiNi0.5Mn1.5O4 with ordered or disordered structures. J Alloys Compd 749:1009–1018. https://doi.org/10.1016/j.jallcom.2018.03.177
Nageswaran S, Keppeler M, Kim SJ, Srinivasan M (2017) Morphology controlled Si-modified LiNi0.5Mn1.5O4 microspheres as high performance high voltage cathode materials in lithium ion batteries. J Power Sources 346:89–96. https://doi.org/10.1016/j.jpowsour.2017.02.013
Liu D, Bai Y, Zhao S, Zhang W (2012) Improved cycling performance of 5 v spinel LiNi0.5Mn1.5O4 by amorphous FePO4 coating. J Power Sources 219:333–338. https://doi.org/10.1016/j.jpowsour.2012.07.058
Wang H, Tan TA, Yang P, et al High rate performances of the Ru doped spinel LiNi0.5Mn1.5O4 : effects of doping and particle size. 4–5
Liu H, Wang J, Zhang X et al (2016) Morphological evolution of high-voltage spinel LiNi0.5Mn1.5O4 cathode materials for lithium-ion batteries: the critical effects of surface orientations and particle size. https://doi.org/10.1021/acsami.5b11389
Park M, Zhang X, Chung M, Less GB, Sastry AM (2010) A review of conduction phenomena in Li-ion batteries. J Power Sources 195:7904–7929. https://doi.org/10.1016/j.jpowsour.2010.06.060
Acknowledgments
Jia guo would like to thank Yecheng Fan from Shiyanjia Lab (www.shiyanjia.com) for the XPS analysis.
Funding
This study received financial support from the Government of Chongzuo, Guangxi Zhuang Autonomous Region (GC Joint Special Fund No. FA2017032) and the kind support from the Science and Technology Department of Guangxi Zhuang Autonomous Region (Guangxi Special Fund for Scientific Center and Talent Resources, No. 2018AD15002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jia Guo will handle correspondence at all stages of refereeing and publication, also post-publication
Electronic supplementary material
ESM 1
(DOC 1443 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Guo, J., Chen, Y. et al. Phase transition regulation and Cd-O/Cd-F compounds multi-effect modification synergistically act on LiNi0.5Mn1.5O4 cathode. Ionics 26, 1681–1693 (2020). https://doi.org/10.1007/s11581-019-03257-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03257-1