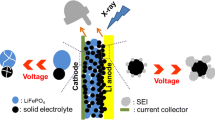
Abstract
We introduce low levels of CsClO4 and RbClO4 into the electrolyte of LiCoO2 electrochemical half-cells to probe the composition of the passivation film on the surface of the cathode, the electrolyte decomposition layer (EDL). The advantages of these heavy alkali dopants lie in their large ionic radii, which limit intercalation, yet their strong light scattering cross-section creates a beacon that highlights the formation of products near the cathode surface. Detailed surface analysis and depth profiling with X-ray photoelectron spectroscopy, and bulk analysis utilizing X-ray absorption spectroscopy, show evidence for the formation of Cs/Rb compounds, such as carbonates, halides, and perchlorates, similar to those formed by lithium in previous studies, but also reveal the significantly reduced mobility of the Cs/Rb relative to Li in the non-uniform EDL. This unique approach could open several presently untapped techniques to gather new information on the EDL in Li-ion batteries.







Similar content being viewed by others
References
Peled E (1979) The electrochemical-behavior of alkali and alkaline-earth metals in non-aqueous battery systems—the Solid Electrolyte Interphase Model. J Electrochem Soc 126(12):2047–2051. doi:10.1149/1.2128859
Lu YC, Mansour AN, Yabuuchi N, Shao-Horn Y (2009) Probing the origin of enhanced stability of "AlPO(4)" nanoparticle coated LiCoO2 during cycling to high voltages: combined XRD and XPS studies. Chem Mat 21(19):4408–4424. doi:10.1021/Cm900862v
Ensling D, Thissen A, Jaegermann W (2008) On the formation of lithium oxides and carbonates on Li metal electrodes in comparison to LiCoO2 surface phases investigated by photoelectron spectroscopy. Appl Surf Sci 255(5):2517–2523. doi:10.1016/j.apsusc.2008.07.196
Malmgren S, Ciosek K, Hahlin M, Gustafsson T, Gorgoi M, Rensmo H, Edstrom K (2013) Comparing anode and cathode electrode/electrolyte interface composition and morphology using soft and hard X-ray photoelectron spectroscopy. Electrochim Acta 97:23–32. doi:10.1016/j.electacta.2013.03.010
Quinlan RA, Lu YC, Shao-Horn Y, Mansour AN (2013) XPS studies of surface chemistry changes of LiNi0.5Mn0.5O2 electrodes during high-voltage cycling. J Electrochem Soc 160(4):A669–A677. doi:10.1149/2.069304jes
Aurbach D, Gamolsky K, Markovsky B, Salitra G, Gofer Y, Heider U, Oesten R, Schmidt M (2000) The study of surface phenomena related to electrochemical lithium intercalation into LixMOy host materials (M = Ni, Mn). J Electrochem Soc 147(4):1322–1331. doi:10.1149/1.1393357
Aurbach D, Markovsky B, Rodkin A, Levi E, Cohen YS, Kim HJ, Schmidt M (2002) On the capacity fading of LiCoO2 intercalation electrodes: the effect of cycling, storage, temperature, and surface film forming additives. Electrochim Acta 47(27):4291–4306. doi:10.1016/S0013-4686(02)00417-6
Verma P, Maire P, Novak P (2010) A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim Acta 55(22):6332–6341. doi:10.1016/j.electacta.2010.05.072
Itagaki M, Kobari N, Yotsuda S, Watanabe K, Kinoshita S, Ue M (2005) LiCoO2 electrode/electrolyte interface of Li-ion rechargeable batteries investigated by in situ electrochemical impedance spectroscopy. J Power Sources 148:78–84. doi:10.1016/j.jpowsour.2005.02.007
Qiu XY, Zhuang QC, Zhang QQ, Cao R, Ying PZ, Qiang YH, Sun SG (2012) Electrochemical and electronic properties of LiCoO2 cathode investigated by galvanostatic cycling and EIS. Phys Chem Chem Phys 14(8):2617–2630. doi:10.1039/C2cp23626e
Nobili F, Dsoke S, Croce F, Marassi R (2005) An ac impedance spectroscopic study of Mg-doped LiCoO2 at different temperatures: electronic and ionic transport properties. Electrochim Acta 50(11):2307–2313. doi:10.1016/j.electacta.2004.10.044
Wang FM, Yu MH, Hsiao YJ, Tsai Y, Hwang BJ, Wang YY, Wan CC (2011) Aging effects to solid electrolyte interface (SEI) membrane formation and the performance analysis of lithium ion batteries. Int J Electrochem Sc 6(4):1014–1026
Huang C, Zhuang S, Tu F (2013) Electrode/electrolyte interfacial behaviors of LiCoO2/mixed graphite Li-ion cells during operation and storage. J Electrochem Soc 160(2):A376–A382. doi:10.1149/2.009303jes
Grey CP, Dupre N (2004) NMR studies of cathode materials for lithium-ion rechargeable batteries. Chem Rev 104(10):4493–4512. doi:10.1021/Cr020734p
Blanc F, Leskes M, Grey CP (2013) In situ solid-state NMR spectroscopy of electrochemical cells: batteries, supercapacitors, and fuel cells. Acc Chem Res 46(9):1952–1963. doi:10.1021/ar400022u
Wang Y, Guo X, Greenbaum S, Liu J, Amine K (2001) Solid electrolyte interphase formation on lithium-ion electrodes: a 7Li nuclear magnetic resonance study. Electrochem Solid-State Lett 4(6):A68–A70
Lu P, Harris SJ (2011) Lithium transport within the solid electrolyte interphase. Electrochem Commun 13(10):1035–1037. doi:10.1016/j.elecom.2011.06.026
Patridge CJ, Love CT, Swider-Lyons KE, Twigg ME, Ramaker DE (2013) In-situ X-ray absorption spectroscopy analysis of capacity fade in nanoscale-LiCoO2. J Solid State Chem 203:134–144. doi:10.1016/j.jssc.2013.04.008
Balasubramanian M, Lee HS, Sun X, Yang XQ, Moodenbaugh AR, McBreen J, Fischer DA, Fu Z (2002) Formation of SEI on cycled lithium-ion battery cathodes—soft X-ray absorption study. Electrochem Solid St 5(1):A22–A25
Love CT, Korovina A, Patridge CJ, Swider-Lyons KE, Twigg ME, Ramaker DE (2013) Review of LiFePO4 phase transition mechanisms and new observations from X-ray absorption spectroscopy. J Electrochem Soc 160(5):A3153–A3161. doi:10.1149/2.023305jes
McBreen J, Balasubramanian M, Yang X, Lee H, Yoon W, Moodenbaugh A, Fischer D, Fu Z, Abraham D Synchrotron X-ray studies of the solid electrolyte interface on cycled lithium-ion battery cathodes. In: Power sources for transportation applications: proceedings of the international symposium, 2004. The Electrochemical Society, p 96
Zhang HL, Li F, Liu C, Tan J, Cheng HM (2005) New insight into the solid electrolyte interphase with use of a focused ion beam. J Phys Chem B 109(47):22205–22211. doi:10.1021/Jp053311a
Wang ZX, Huang XJ, Chen LQ (2004) Characterization of spontaneous reactions of LiCoO2 with electrolyte solvent for lithium-ion batteries. J Electrochem Soc 151(10):A1641–A1652. doi:10.1149/1.1793651
Lei JL, Li LJ, Kostecki R, Muller R, McLarnon F (2005) Characterization of SEI layers on LiMn2O4 cathodes with in situ spectroscopic ellipsometry. J Electrochem Soc 152(4):A774–A777. doi:10.1149/1.1867652
Shi SQ, Lu P, Liu ZY, Qi Y, Hector LG, Li H, Harris SJ (2012) Direct calculation of Li-Ion transport in the solid electrolyte interphase. J Am Chem Soc 134(37):15476–15487. doi:10.1021/Ja305366r
Leung K (2013) Electronic structure modeling of electrochemical reactions at electrode/electrolyte interfaces in lithium ion batteries. J Phys Chem C 117(4):1539–1547. doi:10.1021/Jp308929a
Kim SP, van Duin ACT, Shenoy VB (2011) Effect of electrolytes on the structure and evolution of the solid electrolyte interphase (SEI) in Li-ion batteries: a molecular dynamics study. J Power Sources 196(20):8590–8597. doi:10.1016/j.jpowsour.2011.05.061
Lu P, Li C, Schneider EW, Harris SJ (2014) Chemistry, impedance, and morphology evolution in solid electrolyte interphase films during formation in lithium ion batteries. J Phys Chem C 118(2):896–903. doi:10.1021/Jp4111019
Troltzsch U, Kanoun O, Trankler HR (2006) Characterizing aging effects of lithium ion batteries by impedance spectroscopy. Electrochim Acta 51(8–9):1664–1672
Pinson MB, Bazant MZ (2013) Theory of SEI formation in rechargeable batteries: capacity fade, accelerated aging and lifetime prediction. J Electrochem Soc 160(2):A243–A250. doi:10.1149/2.044302jes
Zhang SS (2006) A review on electrolyte additives for lithium-ion batteries. J Power Sources 162(2):1379–1394. doi:10.1016/j.jpowsour.2006.07.074
Ding F, Xu W, Graff GL, Zhang J, Sushko ML, Chen X, Shao Y, Engelhard MH, Nie Z, Xiao J, Liu X, Sushko PV, Liu J, Zhang J-G (2013) Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J Am Chem Soc 135(11):4450–4456. doi:10.1021/ja312241y
Takeuchi S, Fukutsuka T, Miyazaki K, Abe T (2013) Electrochemical lithium ion intercalation into graphite electrode in propylene carbonate-based electrolytes with dimethyl carbonate and calcium salt. J Power Sources 238:65–68. doi:10.1016/j.jpowsour.2013.02.084
Ravel B, Newville M (2005) ATHENA and ARTEMIS: Interactive graphical data analysis using IFEFFIT. Phys Scr T115:1007–1010
Edström K, Gustafsson T, Thomas JO (2004) The cathode–electrolyte interface in the Li-ion battery. Electrochim Acta 50(2–3):397–403. doi:10.1016/j.electacta.2004.03.049
Cherkashinin G, Nikolowski K, Ehrenberg H, Jacke S, Dimesso L, Jaegermann W (2012) The stability of the SEI layer, surface composition and the oxidation state of transition metals at the electrolyte-cathode interface impacted by the electrochemical cycling: X-ray photoelectron spectroscopy investigation. Phys Chem Chem Phys 14(35):12321–12331. doi:10.1039/C2cp41134b
Morgan WE, Stec WJ, Vanwazer JR (1973) Inner-orbital photoelectron spectroscopy of alkali-metal halides, perchlorates, phosphates, and pyrophosphates. J Am Chem Soc 95(3):751–755. doi:10.1021/Ja00784a018
Shchukarev AV, Korolkov DV (2004) XPS study of group IA carbonates. Cent Eur J Chem 2(2):347–362. doi:10.2478/Bf02475578
Verdier S, El Ouatani L, Dedryvere R, Bonhomme F, Biensan P, Gonbeau D (2007) XPS study on Al2O3- and AlPO4-coated LiCoO2 cathode material for high-capacity li ion batteries. J Electrochem Soc 154(12):A1088–A1099. doi:10.1149/1.2789299
Eriksson T, Andersson AM, Bishop AG, Gejke C, Gustafsson T, Thomas JO (2002) Surface analysis of LiMn2O4 electrodes in carbonate-based electrolytes. J Electrochem Soc 149(1):A69–A78. doi:10.1149/1.1426398
Daheron L, Martinez H, Dedryvere R, Baraille I, Menetrier M, Denage C, Delmas C, Gonbeau D (2009) Surface properties of LiCoO2 investigated by XPS analyses and theoretical calculations. J Phys Chem C 113(14):5843–5852. doi:10.1021/Jp803266w
Daheron L, Dedryvere R, Martinez H, Menetrier M, Denage C, Delmas C, Gonbeau D (2008) Electron transfer mechanisms upon lithium deintercalation from LiCoO2 to CoO2 investigated by XPS. Chem Mat 20(2):583–590. doi:10.1021/Cm702546s
Martin-Vosshage D, Chowdari BVR (1995) XPS studies on (PEO)n LiClO4 and (PEO)n Cu(ClO4) 2 polymer electrolytes. J Electrochem Soc 142(5):1442–1446. doi:10.1149/1.2048594
Brown G, Gu B (2006) The chemistry of perchlorate in the environment. In: Gu B, Coates J (eds) Perchlorate. Springer, US, pp 17–47. doi:10.1007/0-387-31113-0_2
Herstedt M, Stjerndahl M, Nyten A, Gustafsson T, Rensmo H, Siegbahn H, Ravet N, Armand M, Thomas JO, Edstrom K (2003) Surface chemistry of carbon-treated LiFePO4 particles for Li-ion battery cathodes studied by PES. Electrochem Solid St 6(9):A202–A206. doi:10.1149/1.1594413
Tardy Y, Gartner L (1977) Relationships among Gibbs energies of formation of sulfates, nitrates, carbonates, oxides and aqueous ions. Contrib Miner Petr 63(1):89–102. doi:10.1007/Bf00371678
Aurbach D, Daroux ML, Faguy PW, Yeager E (1987) Identification of surface-films formed on lithium in propylene carbonate solutions. J Electrochem Soc 134(7):1611–1620. doi:10.1149/1.2100722
Xu K (2004) Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev 104(10):4303–4417. doi:10.1021/Cr030203g
Seah MP, Dench WA (1979) Quantitative electron spectroscopy of surfaces: a standard data base for electron inelastic mean free paths in solids. Surf Interface Anal 1(1):2–11. doi:10.1002/sia.740010103
Powell CJ, Jablonski A (1999) Evaluation of calculated and measured electron inelastic mean free paths near solid surfaces. J Phys Chem Ref Data 28(1):19–62. doi:10.1063/1.556035
Scofield JH (1973) Theoretical photoionization cross sections from 1 to 1500 keV. Other Information: UNCL. Orig. Receipt Date: 30-JUN-73
Paynter RW, Edgell MJ, Castle JE (1986) The use of monochromatic Ag L-Alpha radiation to study relaxation energy differences in X-ray photoelectron-spectroscopy of alkali-metal chlorides. J Electron Spectrosc 40(1):1–9. doi:10.1016/0368-2048(86)80002-0
Acknowledgments
The authors are grateful to the Office of Naval Research for support of this work through the Naval Research Laboratory. CP would like to thank the National Research Council for financial support and Dr. Bradford Pate for assistance and guidance in collecting and analyzing the XPS measurements. The synchrotron measurements were successful due to the help of Dr. Kumi Pandya. The National Synchrotron Light Source is supported by the U.S. Department of Energy, Division of Material Sciences and Division of Chemical Sciences, under contract number DE-AC02-98CH10886. The X11 beamline is supported by the U.S. Naval Research Laboratory and contributions from Participating Research Team (PRT) members.
Supporting information
XPS elemental scans, electrochemical data, XANES spectra, and XANES standards.
Funding sources
This work was funded by the Office of Naval Research through the Naval Research Laboratory.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1270 kb)
Rights and permissions
About this article
Cite this article
Patridge, C.J., Love, C.T. & Ramaker, D.E. Utilization of heavy alkali dopants as a beacon to study the cathode electrolyte decomposition layer in lithium-ion batteries. Ionics 22, 51–62 (2016). https://doi.org/10.1007/s11581-015-1519-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1519-7