Abstract
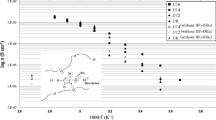
The present work is an effort to study the effects of Li doping on the structural and transport properties of the solid polymer electrolyte, poly-ethelene oxide (PEO) (molecular weight, 200,000). Li-doped PEO was synthesized by treating PEO with n-Butyllithium in hexane for different doping concentrations. It is seen that the crystallinity of the doped PEO decreases on increasing the Li doping concentration and XRD and FTIR studies support this observation. FESEM images give better details of surface morphology of doped PEO samples. The TGA curves of PEO and Li-doped PEO samples reveal the weight loss region, and it is observed that the weight loss process of the solid polymer electrolyte is gradual rather than abrupt, contrary to the case of liquid electrolytes. The purity and the electrochemical stability of the samples were established by cyclic voltammetry studies. Impedance measurements were carried out to estimate the ionic conductivity of Li-doped PEO samples. The present value of ionic conductivity observed at room temperature in Li-doped PEO is about five orders higher than that of pure PEO and is quite close to that of liquid electrolytes. It is inferred that, ionic conductivity of the sample is increasing on increasing the Li doping concentration due to enhanced charge carrier density and flexibility of the doped sample structure. The ionic mobility and ionic transport are significantly improved by the less crystallinity and higher flexibility of the Li-doped PEO samples which in turn are responsible for the enhanced ionic conductivity observed.







Similar content being viewed by others
References
Scrosati B (1993) Application of electroactive polymers. Chapman and Hall, London
Gray FM (1997) Polymer electrolytes. The Royal Society of Chemistry, Letchworth
Ibrahim S, Johan MR (2012) Int J Electrochem Sci 7:2596–2615
Fergus JW (2010) Rev J Power Sources 195:4554–4569
Scrosati B, Garche J (2010) J Power Sources 195:2419–2430
Goodenough JB, Kim Y (2010) Chem Mater 22(3):587–603
Thangadurai V, Weppner W (2006) Ionics 12:81–92
Thangadurai V, Weppner W (2002) Ionics 8:281–292
Knauth P (2009) Solid State Ionics 180:911–916
Anurova NA, Blatov VA, Ilyushin GD, Blatova OA, Ivanov-Schitz AK, Dem’yanets LN (2008) Solid State Ionics 179:2248–2254
Ivanov-Shitz AK (2007) Crystall Rep 52(2):302–315
Gray F, Armand M (2000) In: Osaka T, Datta M (eds) Energy storage systems for electronics. Gordon and Breach Sci. Publ, Amsterdam, pp 351–406
Ahmad S (2009) Ionics 15:309–321
Song JY, Wang YY, Wan CC, Power J (1999) Sources 77:183–197
Stephan AM, Nahm KS (2006) Polymer 47:5952–5964
Appetecchi GB, Montanino M, Balducci A, Simon FL, Winterb M, Passerini S (2009) J Power Sources 192:599
Vuia RA, Visudeevan S, Krawiec W, Scunlon LG, Giannelis EP (1995) Adv Mater 7:2
Varma SJ et al (2012) Polym Int 61:743–748
Sinha S, Bhadra S, Khastgir D (2009) J Appl Polym Sci 112:3135–3140
Da Costa VM, Fiske TG, Coleman LB (1994) Far infrared reflection. J Chem Phys 101:2746
Yoshihara T, Tadokoro H, Murahashi S (1964) J Chern Phys 41:2902
Kong Y, Hay JN (2002) Polymer 43:3873
Lee YS, Lee WK, Cho SG II, Kim CSH (2007) J Anal Appl Pyrolysis 78:85
Pradhan DK, Choudhary RNP, Samantaray BK (2008) Int J Electrochem Sci 3:597–608
Ibrahim S et al (2012) Solid State Commun 152:426–434
Li N, Wang L, He X, Wan C, Jiang C (2008) Ionics 14:463–467
An SY, Jeong IC, Won M-S, Jeong ED, Shim Y-B (2009) J Appl Electrochem 39:1573–1578
Derrien G, Hassoun J, Sacchetti S, Panero S (2009) Solid State Ionics 180:1267–1271
Shen C, Wang J, Tang Z, Wang H, Lian H, Zhang J, Cao C-N (2009) Electrochim Acta 54:3490–3494
Sumathipala HH, Hassoun J, Panero S, Scrosati B (2007) Ionics 13:281–286
Acknowledgments
The financial assistance provided by the Board of Research in Nuclear Sciences (BRNS), Government of India, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puthirath, A.B., John, B., Gouri, C. et al. Lithium-doped PEO—a prospective solid electrolyte with high ionic conductivity, developed using n-Butyllithium in hexane as dopant. Ionics 21, 2185–2191 (2015). https://doi.org/10.1007/s11581-015-1406-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1406-2