Abstract
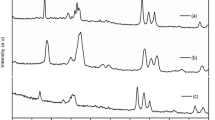
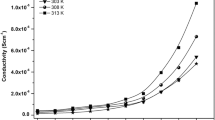
Nanocomposite polymer electrolyte (NCPE) films based on polyethylene oxide (PEO) complexed with lithium perchlorate (LiClO4) and nanosized ferroelectric ceramic fillers such as BaTiO3, SrTiO3 have been prepared using solution cast technique. The films showed very good mechanical stability when exposed to ambient atmospheres for prolonged periods. Lithium ion transport studies revealed that the conductivity is predominantly ionic. The effect of electric field on ionic conductivity of NCPE films was investigated. One order enhancement in conductivity due to the field was observed at 323 K. NCPE films exhibited conductivity of 3.46 × 10−5 Scm−1 at 323 K. NCPE films were characterized using differential scanning calorimetry (DSC) and X-ray diffraction (XRD) technique. The DSC and XRD studies revealed reduced crystallinity which confirmed the higher amorphous phase and hence the improved ionic conductivity.








Similar content being viewed by others
References
Felix BD, Lambertus P, Jakobert BJ, Veldhuis (2000) J Power Sources 88:169–191
Ludvig E, Marca MD (2003) Solid State Ionics 158:177–186
Hikosaka MY, Pulcinelli SH, Santilli CV, Dahmouche K, Craievich AF (2006) J Non-Cryst Solids 352:3705–3710
Manoj K, Sekhon SS (2002) Ionics 8:223–233
Sumathipala HH, Hassoun J, Panero S, Scrosati B (2007) Ionics 13:281–286
Lakshman Dissanayake MAK (2004) Ionics 10:221–225
Hashmi SA, Upadhaya HM, Thakur AK, Verma AL (2000) Ionics 6:248–259
Ragavendran K, Kalyani P, Veluchamy A, Banumathi S, Thirunakaran R, Benidict TJ (2004) Port Electrochim Acta 22:149–159
Shanmukaraj D, Wang GX, Liu HK, Murugan R (2008) Polymer Bulletin; 351–361
Ratna D, Divekar S, Samui AB, Chakraborty BC, Banthia AK (2006) Polymer 47:4068–4074
Kulia T, Acharya H, Srivastava SK, Samantaray BK, Kureti S (2007) Mater Sci Eng B 137:217–224
Fullerton-shirey SK, Janna KM (2010) J Phys Chem C 114:9196–9206
Al F, D’Aprea A, Nadia EK, Alain D, Frederic B (2010) Electrochim Acta 55:5186–5194
Palanisamy V, Dhandapani R (2011) Ionics 17:565–571
Sasikala U, Naveen KP, Rao VVRN, Sharma AK (2012) Inter J Eng Sci Adv Technol 2P:722–730
Vijay KK, Suneeta SKG (2010) Inter J Eng Sci Adv Technol 5:130–139
Lizhen F, Ce-Wen N, Ming L (2003) Chem Phys Lett 369:698–702
Chung SH, Wang Y, Persi L, Croce F, Greenbaum SG, Scrosati B, Plichta E (2001) J Power Sources 97–98:644–648
Ahmad A, K.B Md.Isa KB, Osman Z (2011) Sains Malaysiana 40(7): 691–694
Chien-Shiun L, Wei-Bin Y (2003) J Polymer Res 10:241–246
Ji J, Li B, Zhong W-H (2010) Electrochim Acta 55:9075–9082
Suriani I, Mohd Rafie J (2012) Int J Electrochem Sci 7:2596–2615
Siti Aminah bt, Azizan Ahmad, Mohd. Yusri bin Abd. Rahaman, Ibrahim Abu Talib Talib (2010) Natural science 2; 190–196
Acknowledgments
The authors thank Visvesvaraya Technological University, Belgaum, India for the financial support for this work under research project grant. The authors also thank Dr. Praveen C. Ramamurthy, Indian Institute of Science, Bangalore, for facilitating the XRD studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sunitha V. R., Radhakrishnan S. Field enhanced Li ion conduction in nanoferroelectric modified polymer electrolyte systems. Ionics 21, 949–954 (2015). https://doi.org/10.1007/s11581-014-1252-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1252-7