Abstract
Mg-7.5Li-3.5Al and Mg-7.5Li-3.5Al-1Y alloys are prepared, and their electrochemical performances are investigated by potentiodynamic polarization, potentiostatic current-time curves, electrochemical impedance spectroscopy, scanning electron microscopy (SEM), varying load performances, and utilization efficiencies. The performances of the Mg-H2O2 semi-fuel cells with the above alloy anodes are also determined. It is found that the Mg-7.5Li-3.5Al-1Y electrode has higher discharge activity and better corrosion resistance than Mg-7.5Li-3.5Al electrode in 0.7 mol L−1 NaCl solution. SEM studies indicate that the alloying element Y prevents the formation of dense oxide film on the alloy surface and facilitates peeling off of the oxidation products. The utilization efficiencies of the alloys increase in the order: Mg-7.5Li-3.5Al < Mg-7.5Li-3.5Al-1Y. The Mg-H2O2 semi-fuel cell with Mg-7.5Li-3.5Al-1Y alloy as the anode presents a maximum power density of 92 mW cm−2 at room temperature, which is higher than that with Mg-7.5Li-3.5Al anode (85 mW cm−2). Y is a “promotion element” in the alloy, and the Mg-7.5Li-3.5Al-1Y alloy electrode exhibits better performances comparing to the Mg-7.5Li-3.5Al alloy electrode.

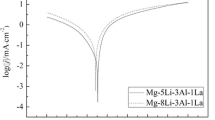
The Mg-7.5Li-3.5Al-1Y electrode shows better discharge performances than that of the Mg-7.5Li-3.5-Al electrode at the same discharging potential.






Similar content being viewed by others
References
Hasvold O, Storkersen NJ, Forseth S, Lian T (2006) Power sources for autonomous underwater vehicles. J Power Sources 162:935–942
Medeiros MG, Bessette RR, Deschenes CM, Patrissi CJ, Carreiro LG, Tucker SP, Atwater DW (2004) Magnesium-solution phase catholyte semi-fuel cell for undersea vehicles. J Power Sources 136:226–2311
Bessette RR, Medeiros MG, Patrissi CJ, Deschenes CM, LaFratta CN (2001) Development and characterization of a novel carbon fiber based cathode for semi-fuel cell applications. J Power Sources 96:240–244
Bessette RR, Cichon JM, Dischert DW, Dow EG (1999) A study of cathode catalysis for the aluminium/hydrogen peroxide semi-fuel cell. J Power Sources 80:248–253
Yang WQ, Yang SH, Sun W, Sun GQ, Xin Q (2006) Nanostructured silver catalyzed nickel foam cathode for an aluminum-hydrogen peroxide fuel cell. J Power Sources 160:1420–1424
Yang WQ, Yang SH, Sun W, Sun GQ, Xin Q (2006) Nanostructured palladium-silver coated nickel foam cathode for magnesium-hydrogen peroxide fuel cells. Electrochim Acta 52:9–14
Brodrecht DJ, Rusek JJ (2003) Aluminum–hydrogen peroxide fuel-cell studies. Appl Energy 74:113–124
Medeiros MG, Zoski CG (1998) Investigation of a sodium hypochlorite catholyte for an aluminum aqueous battery system. J Phys Chem B 102:9908–9914
Ono S, Asami K, Osaka T, Masuko N (1996) Structure of anodic films on magnesium. J Electrochem Soc 143:106–109
Song DL, Jing XY, Wang J (2011) Microwave-assisted synthesis of lanthanum conversion coating on Mg–Li alloy and its corrosion resistance. Corros Sci 53:3651–3656
Yang WQ, Yang SH, Sun W (2006) Nanostructured palladium-silver coated nickel foam cathode for magnesium–hydrogen peroxide fuel cells. Electrochim Acta 52:9–14
Medeiros MG, Bessette RR, Deschenes CM, Atwater DW (2001) Optimization of the magnesium-solution phase catholyte semi-fuel cell for long duration testing. J Power Sources 96:236–239
Medeiros MG, Dow EG (1999) Magnesium-solution phase catholyte seawater electrochemical system. J Power Sources 80:78–82
Sivashanmugam A, Kumar TP, Renganathan NG, Gopukumarl S (2004) Performance of a magnesium-lithium alloy as an anode for magnesium batteries. J Appl Electrochem 34:1135–1139
Hamlen RP, Atwater DW (2002) in Handbook of batteries, ed. Linden D, Reddy TB, McGraw-Hill, 3rd edn, p. 381
Cao DX, Wu L, Sun Y, Wang GL, Lv YZ (2008) Electrochemical behavior of Mg-Li, Mg-Li-Al and Mg-Li-Al-Ce in sodium chloride solution. J Power Sources 177:624–630
Lv YZ, Liu M, Xu Y, Cao DX, Feng J (2013) The electrochemical behaviors of Mge-8Li-3Al-0.5Zn and Mg-8Li-3Al-1.0Zn in sodium chloride solution. J Power Sources 225:124–128
Lv YZ, Jin YZ, Wang ZB, Li YF, Wang L, Cao DX (2014) The effect of sodium stannate as the electrolyte additive on the electrochemical performances of the Mg-8Li-1Y electrode in NaCl solution. RSC Adv 4:18074–18079
Kumar BVR, Sathyanarayana S (1983) The delayed action of magnesium anodes in primary batteries Part I. Experimental studies. J Power Sources 10:219–241
Sathyanarayana S, Kumar BVR (1983) The delayed action of magnesium anodes in primary batteries: Part II. Theoretical studies. J Power Sources 10:243–261
Song G, Atrens A, John DS, Wu X, Nairn J (1997) The anodic dissolution of magnesium in chloride and sulphate solutions. Corros Sci 39:1981–2004
Anik M, Celikten G (2007) Analysis of the electrochemical reaction behavior of alloy AZ91 by EIS technique in H3PO4/KOH buffered K2SO4 solutions. Corros Sci 49:1878–1894
Baril G, Blanc C, Pebere N (2001) Impedance spectroscopy in characterizing time-dependent corrosion of AZ91 and AM50 magnesium alloys characterization with respect to their microstructures. J Electrochem Soc 148:489–496
Baril G, Pebere N (2001) The corrosion of pure magnesium in aerated and deaerated sodium sulphate solutions. Corros Sci 43:471–484
Lv YZ, Liu M, Xu Y, Cao DX, Feng J, Wu RZ, Zhang ML (2013) The electrochemical behaviors of Mg-8Lie-0.5Y and Mg-8Li-1Y alloys in sodium chloride solution. J Power Sources 239:265–268
Lv YZ, Xu Y, Cao DX (2011) The electrochemical behaviors of Mg, Mg-Li-Al-Ce and Mg-Li-Al-Ce-Y in sodium chloride solution. J Power Sources 196:8809–8814
Acknowledgments
We gratefully acknowledge the Natural Science Foundation of Heilongjiang Province of China (B201201), the National Natural Science Foundation of China (21203040, 21301038, 51108111), and the Fundamental Research Funds for the Central Universities (HEUCF201403018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lv, YZ., Jin, YZ., Wang, ZB. et al. The electrochemical behaviors of the Mg-7.5Li-3.5Al and Mg-7.5Li-3.5Al-1Y electrodes in sodium chloride solution. Ionics 21, 429–435 (2015). https://doi.org/10.1007/s11581-014-1187-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1187-z