Abstract
Purpose
To assess the correlation between functional MRI, including ADC values obtained from DWI and DCE, and clinical outcome in patients with bone metastases treated with MRgFUS.
Methods and materials
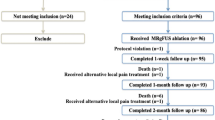
Twenty-three patients with symptomatic bone metastases underwent MRgFUS treatment (ExAblate 2100 system InSightec) for pain palliation. All patients underwent clinical and imaging follow-up examinations at 1, 3 and 6 months after treatment. Visual Analog Scale (VAS) score was used to evaluate treatment efficacy in terms of pain palliation while ADC maps obtained by DWI sequences, and DCE data were used for quantitative assessment of treatment response at imaging. Spearman Correlation Coefficient Test was calculated to assess the correlation between VAS, ADC and DCE data.
Results
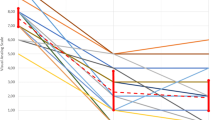
All treatments were performed successfully without adverse events. On the basis of VAS score, 16 (69.6 %) patients were classified as complete clinical responders, 6 (26.1 %) as partial responders and only one (4.3 %) was classified as a non-responder. The mean VAS score decreased from 7.09 ± 1.8 at baseline to 2.65 ± 1.36 at 1 month, 1.04 ± 1.91 at 3 months and 1.09 ± 1.99 at 6 months (p < 0.001). Baseline mean ADC value of treated lesions was 1.05 ± 0.15 mm2/s, increasing along follow-up period (1.57 ± 0.27 mm2/s 1st month; 1.49 ± 0.3 mm2/s 3rd month; 1.45 ± 0.32 mm2/s 6th month, p < 0.001). Non perfused volume (NPV) was 46.4 at 1 month, 45.2 at 3 months and 43.8 at 6 months. Spearman Coefficient demonstrated a statistically significant negative correlation between VAS and ADC values (ρ = −0.684; p = 0.03), but no significant correlation between VAS and NPV (ρ = 0.02216, p = 0.9305). Among other DCE data, Ktrans significantly changed in complete responders (3 months Ktrans = 2.14/min; −ΔKt = 52.65 % p < 0.01) and was not significantly different in partial responders (3 months Ktrans 0.042/min; ΔKt = 11.39 % p > 0.01).
Conclusion
In patients with painful bone metastases treated with MRgFUS, ADC and Ktrans variation observed in the ablated lesions correlate with VAS values and may play a role as objective imaging marker of treatment response.





Similar content being viewed by others
References
Liberman B, Gianfelice D, Inbar Y et al (2009) Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol 16(1):140–146
Hurwitz MD, Ghanouni P, Kanaev SV et al (2014) Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst 106(5). doi:10.1093/jnci/dju082
Napoli A, Anzidei M, Marincola BC et al (2013) MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics 33(6):1555–1568
Napoli A, Anzidei M, Marincola BC et al (2013) Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound. Invest Radiol 48(6):351–358
Hamaoka T, Costelloe CM, Madewell JE et al (2010) Tumour response interpretation with new tumour response criteria vs the World Health Organisation criteria in patients with bone-only metastatic breast cancer. Br J Cancer 102(4):651–657
Saip P, Tenekeci N, Aydiner A et al (1999) Response evaluation of bone metastases in breast cancer: value of magnetic resonance imaging. Cancer Invest 17(8):575–580
Wachenfeld I, Sanner G, Böttcher HD, Kollath J (1996) The remineralization of the vertebral metastases of breast carcinoma after radiotherapy. Strahlenther Onkol 172(6):332–341
Vassiliou V, Andreopoulos D, Frangos S, Tselis N, Giannopoulou E, Lutz S (2011) Bone metastases: assessment of therapeutic response through radiological and nuclear medicine imaging modalities. Clin Oncol (R Coll Radiol) 23(9):632–645
Gaeta M, Benedetto C, Minutoli F et al (2014) Use of diffusion-weighted, intravoxel incoherent motion, and dynamic contrast-enhanced MR imaging in the assessment of response to radiotherapy of lytic bone metastases from breast cancer. Acad Radiol 21(10):1286–1293
Sharma P, Karunanithi S, Singh Dhull V, Jain S, Bal C, Kumar R (2013) Prostate cancer with lytic bone metastases: 18F-fluorodeoxyglucose positron emission tomography-computed tomography for diagnosis and monitoring response to medical castration therapy. Indian J Nucl Med 28(3):178–179
Correa PD, Shrimali RK, Han S, Rizwanullah M (2010) Positron emission tomography with computed tomography (PET-CT) to evaluate the response of bone metastases to non-surgical treatment. BMJ Case Rep 2010. doi:10.1136/bcr.11.2009.2457
Fraioli F, Anzidei M, Serra G et al (1029) Whole-tumour CT-perfusion of unresectable lung cancer for the monitoring of anti-angiogenetic chemotherapy effects. Br J Radiol 2013(86):20120174
Low RN, Gurney J (2007) Diffusion-weighted MRI (DWI) in the oncology patient: value of breathhold DWI compared to unenhanced and gadolinium-enhanced MRI. J Magn Reson Imaging 25(4):848–858
Li XR, Cheng LQ, Liu M et al (2012) DW-MRI ADC values can predict treatment response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. Med Oncol 29(2):425–431
Musio D, De Felice F, Magnante AL et al (2013) Diffusion-weighted magnetic resonance application in response prediction before, during, and after neoadjuvant radiochemotherapy in primary rectal cancer carcinoma. Biomed Res Int 2013:740195
Yankeelov TE, Lepage M, Chakravarthy A, Broome EE, Niermann KJ, Kelley MC, Meszoely I, Mayer IA, Herman CR, McManus K, Price RR, Gore JC (2007) Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Magn Reson Imaging 25(1):1–13
Lecouvet FE, Larbi A, Pasoglou V, Omoumi P, Tombal B, Michoux N, Malghem J, Lhommel R, Vande Berg BC (2013) MRI for response assessment in metastatic bone disease. Eur Radiol 23(7):1986–1997
Napoli A, Mastantuono M, Cavallo Marincola B, Anzidei M, Zaccagna F, Moreschini O, Passariello R, Catalano C (2013) Osteoid osteoma: MR-guided focused ultrasound for entirely noninvasive treatment. Radiology 267(2):514–521
Wasserman J, De La Lande B, Pecking A, Brasseur L (2008) Pain and bone metastases. Prog Urol 18(suppl 7):S399–S409
Padhani AR (2011) Bony metastases: assessing response to therapy with whole-bodydiffusion MRI. Cancer Imaging 11(1A):S129–S145
Biffar A, Dietrich O, Sourbron S, Duerr HR, Reiser MF, Baur-Melnyk A (2010) Diffusion and perfusion imaging of bone marrow. Eur J Radiol 76:323–328
Vilanova JC, Baleato-Gonzalez S, Romero MJ, Carrascoso-Arranz J, Luna A (2016) Assessment of musculoskeletal malignancies with functional MR imaging. Magn Reson Imaging Clin N Am 24(1):239–259
Gaeta M, Benedetto C, Minutoli F, D’Angelo T, Amato E, Mazziotti S, Racchiusa S, Mormina E, Blandino A, Pergolizzi S (2014) Use of diffusion-weighted, intravoxel incoherent motion, and dynamic contrast-enhanced MR imaging in the assessment of response to radiotherapy of lytic bone metastases from breast cancer. Acad Radiol 21(10):1286–1293
Larbi A, Dallaudière B, Pasoglou V, Padhani A, Michoux N, Vande Berg BC (2016) Tombal B3, Lecouvet FE. Whole body MRI (WB-MRI) assessment of metastatic spread in prostate cancer: therapeutic perspectives on targeted management of oligometastatic disease. Prostate 76(11):1024–1033
Anzidei M, Napoli A, Zaccagna F, Cartocci G, Saba L, Menichini G, Cavallo Marincola B, Marotta E, Di Mare L, Catalano C, Passariello R (2011) Liver metastases from colorectal cancer treated with conventional and antiangiogenetic chemotherapy: evaluation with liver computed tomography perfusion and magnetic resonance diffusion-weighted imaging. J Comput Assist Tomogr 35(6):690–696
Geith T, Biffar A, Schmidt G, Sourbron S, Dietrich O, Reiser M, Baur-Melnyk A (2015) Physiological background of differences in quantitative diffusion-weighted magnetic resonance imaging between acute malignant and benign vertebral body fractures: correlation of apparent diffusion coefficient with quantitative perfusion magnetic resonance imaging using the 2-compartment exchange model. J Comput Assist Tomogr 39(5):643–648
Blackledge MD, Collins DJ, Tunariu N, Orton MR, Padhani AR, Leach MO, Koh DM (2014) Assessment of treatment response by total tumor volume and global apparent diffusion coefficient using diffusion-weighted MRI in patients with metastatic bone disease: a feasibility study. PLoS One 9(4):e91779
Jambor I, Kuisma A, Ramadan S, Huovinen R, Sandell M, Kajander S, Kemppainen J, Kauppila E, Auren J, Merisaari H, Saunavaara J, Noponen T, Minn H, Aronen HJ, Seppänen M (2016) Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5TMRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol 55(1):59–67
Chenevert TL, McKeever PE, Ross BD (1997) Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res 3(9):1457–1466
Galons JP, Altbach MI, Paine-Murrieta GD, Taylor CW, Gillies RJ (1999) Early increases in breast tumor xenograft water mobility in response to paclitaxel therapy detected by non-invasive diffusion magnetic resonance imaging. Neoplasia 1(2):113–117
Bonekamp S, Corona-Villalobos CP, Kamel IR (2012) Oncologic applications of diffusion-weighted MRI in the body. J Magn Reson Imaging 35(2):257–279
Reischauer C, Froehlich JM, Koh DM et al (2010) Bone metastases from prostate cancer: assessing treatment response by using diffusion-weighted imaging and functional diffusion maps–initial observations. Radiology 257(2):523–531
Mardor Y, Pfeffer R, Spiegelmann R et al (2003) Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J Clin Oncol 21(6):1094–1100
Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, Doran S (2002) Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 360(9329):307–308
DeVries AF, Kremser C, Hein PA et al (2003) Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int J Radiat Oncol Biol Phys 56(4):958–965
Theilmann RJ, Borders R, Trouard TP et al (2004) Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia 6(6):831–837
Woodhams R, Matsunaga K, Iwabuchi K et al (2005) Diffusion-weighted imaging of malignant breast tumors: the usefulness of apparent diffusion coefficient (ADC) value and ADC map for the detection of malignant breast tumors and evaluation of cancer extension. J Comput Assist Tomogr 29(5):644–649
Biffar A, Sourbron S, Schmidt G, Ingrisch M, Dietrich O, Reiser MF, Baur-Melnyk A (2010) Measurement of perfusion and permeability from dynamic contrast-enhanced MRI in normal and pathological vertebral bone marrow. Magn Reson Med 64(1):115–124
O’Connor JP, Jackson A, Parker GJ, Jayson GC (2007) DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer 96(2):189–195
Bäuerle T, Merz M, Komljenovic D, Zwick S, Semmler W (2010) Drug-induced vessel remodeling in bone metastases as assessed by dynamic contrast enhanced magnetic resonance imaging and vessel size imaging: a longitudinal in vivo study. Clin Cancer Res 16(12):3215–3225
Zeng L, Chow E, Zhang L et al (2012) Comparison of pain response and functional interference outcomes between spinal and non-spinal bone metastases treated with palliative radiotherapy. Support Care Cancer 20(3):633–639
Matza LS, Fallowfield LJ, Chung KC, Currie BM, Van Brunt K, Patrick DL (2012) Patient-reported outcome instruments used to assess pain and functioning in studies of bisphosphonate treatment for bone metastases. Support Care Cancer 20(4):657–677
Schreuder SM, Lensing R, Stoker J, Bipat S (2015) Monitoring treatment response in patients undergoing chemoradiotherapy for locally advanced uterine cervical cancer by additional diffusion-weighted imaging: a systematic review. J Magn Reson Imaging 42(3):572–594
Reginelli A, Silvestro G, Fontanella G, Sangiovanni A, Conte M, Nuzzo I, Calvanese M, Traettino M, Ferraioli P, Grassi R, Manzo P, Cappabianca S (2016) Validation of DWI in assessment of radiotreated bone metastases in elderly patients. Int J Surg (pii: S1743-9191(16)30176-5)
Smith NB, Temkin JM, Shapiro F, Hynynen K (2001) Thermal effects of focused ultrasound energy on bone tissue. Ultrasound Med Biol 27(10):1427–1433
Acknowledgments
None declared.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Local Institutional Review Board approval.
Consent for publication
Informed consent was obtained for all patients.
Conflict of interest
None declared.
Rights and permissions
About this article
Cite this article
Anzidei, M., Napoli, A., Sacconi, B. et al. Magnetic resonance-guided focused ultrasound for the treatment of painful bone metastases: role of apparent diffusion coefficient (ADC) and dynamic contrast enhanced (DCE) MRI in the assessment of clinical outcome. Radiol med 121, 905–915 (2016). https://doi.org/10.1007/s11547-016-0675-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-016-0675-9