Abstract
Introduction
One of the aims of fetal magnetic resonance imaging (MRI) is to avoid postnatal scanning. However, clinicians sometimes wish to have postnatal confirmation of prenatal findings. This study’s purpose was to check whether there was indeed the added value of neonatal MRI performed in the MR-compatible incubator (INC) after fetal examination.
Materials and methods
Material consists of 25 neonates (14 girls) who underwent prenatal and postnatal MRI in a 1.5 T scanner, the latter in INC. Mean time of prenatal MRI was 30th gestational week, of postnatal MRI—16th day of life.
Results
In 14 cases (56 %) postnatal findings were the same as prenatal ones. In 11 (44 %) postnatal MRI showed some different/new/more precise results, in two the differences were attributed to other factors than the advantage of postnatal MRI over prenatal one. Altogether then postnatal results were partly discordant with prenatal ones in 9/25 cases (36 %).
Conclusions
In most cases there was no added value of postnatal MRI as compared to prenatal one. This value lied in small details that could not have been noticed on prenatal MRI or required contrast medium administration to be noticed. On the other hand, MR examination performed with use of the dedicated neonatal coils in the MR-compatible incubator is a safe and reliable method of visualization of these small details with better spatial resolution thus helping to establish final diagnosis, treatment plan and prognosis.
Similar content being viewed by others
Introduction
One of the aims of fetal magnetic resonance imaging (MRI), especially if it is performed close to term, is to avoid postnatal scanning as it has been documented that in many disorders prenatal MRI can establish the diagnosis and help in the planning of postnatal management, obviating the need for early postnatal imaging [1–3]. However, clinicians in some cases still wish to have postnatal confirmation of prenatal MRI findings. Although the mother’s uterus is a safe “incubator” for the sick fetus, currently we have a secure equivalent of it for a newborn which is the MR-compatible incubator (INC), offering the safe environment during MRI procedure even for unstable neonates [4, 5]. The literature concerning early postnatal MRI in the INC is very scant and devoted to the brain in vast majority of publications. Thanks to the dedicated neonatal coils built in our INC (eight-channel phase-array head coil and whole body twelve-channel phase-array coil consisting of two elements: eight-channel part integrated with the incubator bed and four-channel separate surface coil) we are able to examine not only the neonatal brain but also other organs and systems.
To the best of our knowledge there are no papers in the literature comparing the value of prenatal and postnatal MRI, with the latter performed in this most up-to date equipment. The purpose of this study was to check whether there was indeed the added value of early postnatal MRI performed in the MR-compatible incubator after the fetal examination without the limitation to the head—with respect to the pathology of the whole body.
Materials and methods
The material consists of 27 neonates who underwent pre- and early postnatal MRI in a 1.5 T scanner. Two neonates could not be examined in the incubator due to the size of extracranial abnormality (suboccipital encephalomeningocele and a tongue tumor). Therefore, finally we included 25 babies (14 girls, 11 boys). Prenatal MRI was performed between the gestational ages of 19 and 38 weeks (mean in the 30th gestational week, GW), postnatal MRI between day 1 (day of birth) and the age of 3 months (mean in the 16th day of life). In four cases two prenatal MR examinations were performed.
Prenatal MRI consisted of the following sequences: single-shot fast spin echo/T2-weighted images (SSFSE/T2WI) in axial, sagittal and coronal planes, fast imaging employing steady state acquisition (FIESTA/2D) in three planes as well, fast spin echo/T1WI (FSE/T1WI), gradient echo echo planar imaging (GRE EPI), susceptibility-weighted imaging (SWI) and diffusion-weighted imaging (DWI) in chosen planes, adequate to the diagnostic problem. The parameters used in the prenatal protocol are presented in Table 1.
Sixteen babies were born at term, nine—preterm, at the gestational age between 31 and 37 weeks (mean in the 34th GW). In all cases included, the study was performed with use of a Nomag IC 1.5 incubator, equipped with three coils: an eight-channel, phased-array head coil and a twelve channel phased-array coil for the whole body, consisting of an eight-channel coil integrated in the incubator and a separate four-channel surface coil.
In postnatal scans, the same sequences were used, and additionally, depending on region of interest (head or body), fluid attenuated inversion recovery (FLAIR) and short TI inversion recovery (STIR) were included in the protocol. The parameters used are shown in Table 2.
Results
In 14 cases (56 %) the MRI findings obtained after birth were the same as in the prenatal examination(s). These results are presented in Table 3. In the remaining 11 cases (44 %) postnatal MRI showed some different or new or more precise results as compared to the fetal one: subependymal heterotopia, callosal hypoplasia, agenesis of the anterior commissure, optic nerves hypoplasia and cranial nerves V, VII, VIII emerging together from the brainstem, cleft palate, subcutaneous hemangioma and not the intraosseous one, atretic encephalocele, unicornuate uterus, multiple enhancing nodules as an addition to the enormous cystic tumor in the abdomen and pelvis in a case of blue rubber bleb nevus syndrome (BRBNS). In 1 baby out of these 11 postnatal MRI performed at term equivalent revealed normal brain gyration while at the gestational age of 26 weeks it was delayed by approximately 2 weeks—so it showed normal brain maturation which has been inharmonious earlier rather than the advantage of postnatal MRI over prenatal one. In another baby with clinically evident microcephaly, cerebellar hypoplasia that was diagnosed prenatally was not confirmed as such on postnatal MRI which revealed proportionally small cerebrum and cerebellum. Altogether then the results of postnatal MRI were partly discordant with prenatal one in 9/25 cases which accounts for 36 % of all the cases. Besides in two cases there were additional postnatal findings related most likely to perinatal insult, such as subependymal bleeding and ischemic focus in the brain. The discrepancies between prenatal and postnatal examinations are shown in Table 4.
Discussion
In more than half of our patients the diagnosis was fully and properly established on prenatal MR examination and only confirmed postnatally. These diagnoses included ventriculomegaly/hydrocephalus, aqueductal stenosis, rhombencephalosynapsis, cerebellar hypoplasia, callosal agenesis/hypoplasia, interhemispheric cyst, pericallosal lipoma, gray matter heterotopia, transsphenoidal encephalocele, face tumor, microphtalmia, cleft lip and palate, neck cyst, Chiari II malformation, ectopic kidney and abdominal cyst.
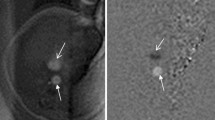
Several studies compared the results of prenatal MRI with postnatal one. Most of them concerned malformations of the central nervous system (CNS) [e.g. 2,6–8] although other systems have also been described [9]. In our material there are also studies of other organs and systems: vertebral column and spinal canal in one case, head and neck in three cases, and abdomen and/or pelvis in three, which accounts for 28 % of the study group. This makes the material inhomogeneous but covers the whole spectrum of MRI utility in both prenatal and postnatal studies. Although congenital abnormalities/damage to CNS still remain the main indication in case of neonatal MR examinations, there is a growing role of this method in body imaging. The technical progress and development of dedicated neonatal body coils which vendors had started to build in the INC allowed evaluation of other parts of the body and not only the brain which is the only organ that has been described in previous studies. Regardless of which organ or system they relate to, prenatal MR examinations allow early planning of postnatal interventions, speeding up the postnatal diagnostic process and minimizing delay in treatment after delivery. In many cases treatment can readily be based on the information available from prenatal imaging [2]. Although fetal MRI has gone far beyond T2-weighted imaging alone and numerous other sequences are available nowadays [10], it still does not have the same diagnostic quality and variety of pulse sequences as are available in the postnatal examination. The use of gadolinium-based contrast media is also reserved for postnatal MRI. Therefore, for example in oncological cases, the clinicians wish to have accurate information about the origin of the tumor, the invasion of adjacent organs, and the presence or absence of metastases after birth. In our case no. 11, we saw prenatally the huge abdominal cyst (Fig. 1a) but only after birth we could appreciate numerous tiny nodules in other localizations that enhanced strongly with contrast medium (Fig. 1b,c). We diagnosed this case as a malignant tumor with metastatic spread but we were wrong as these turned to be venous malformations in BRBNS.
Case no. 11. a Fetal MRI. Huge abdominal and pelvic cyst. b Postnatal MRI. The dominant cyst and a small enhanced nodule in the left lobe of the liver. c Postnatal MRI. Enhanced nodules in the right lobe of the liver behind the main mass, in the chest wall on the right side and in the diaphragm on the left
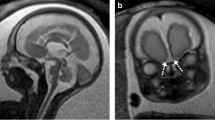
Tiny structures are in general difficult to see on prenatal MRI. They are best seen if changed in a cystic way, due to T2-hyperintensity. Dacryocystocele is a good example. Cranial nerves are in turn a good example of tiny structures which are impossible to assess on prenatal MRI with the exception of the optic nerves which are visible in most fetuses in the second half of pregnancy—in our experience earlier than reported in the literature: according to Brugger, visualization of the optic nerve is associated with change of signal intensity of the retrobulbar adipose tissue, which occurs in the third trimester of pregnancy [11], i.e., since 27th GW. It does not mean, however, that we are able to evaluate whether optic nerves are normal or hypoplastic. So hypoplastic optic nerves and cranial nerves V, VII and VIII emerging together from the brainstem were absolutely impossible to be assessed as such on prenatal MRI in our case no. 14 (Fig. 2a–d). Searching in PubMed for “fetus, cranial nerves, MRI” we have not found any reports concerning fetal MRI in vivo.
Case no. 14. a,b Fetal MRI. T2-weighted images, axial plane. Only deformation of the eye bulbs can be appreciated on these images. c Postnatal MRI. T2-weighted image, axial plane. Bilateral coloboma. d Postnatal MRI. T2-weighted image, axial plane. On the left side cranial nerves V, VII and VIII emerge together from the brain stem. On the right only cranial nerve V is visible in this section
Also subependymal gray matter heterotopia belongs to the list of tiny elements that are difficult to appreciate on prenatal MRI. In our material, it was missed in two cases: no. 3 and 17 (Fig. 3a–b). This is in line with the results of other authors who explain the difficulty in identifying subependymal heterotopia with the fact the nodules have small size and signal intensity similar to the adjacent germinal matrix [8].
If the corpus callosum of the fetus is only hypoplastic, the typical MRI symptoms indicating its agenesis, such as enlargement of the ventricular atria and the occipital horns (colpocephaly), marked separation of the bodies of the lateral ventricles, laterally positioned frontal horns with concave medial borders, elevation of the third ventricle and radial disposition of the sulci on the internal aspects of the hemispheres, may be absent [12]. In our material, this was the case in two fetuses: nos. 14 and 22, and we missed the diagnosis of callosal hypoplasia in these fetuses.
Usually, if we deal with clefts of the primary palate it means cleft lip and alveolus which is diagnosed properly on fetal MRI [13]. In our case no. 22, we diagnosed cleft alveolus on postnatal MRI which was confirmed during surgery. In the literature, we did not find a report on isolated alveolar cleft without cleft lip. The baby was operated on due to the pedunculated fibroma in the mouth with dimensions of 15 × 7 × 7 mm after birth, which was not diagnosed on prenatal MRI either (Fig. 4a–c). It was hard to see postnatally when the newborn kept the mouth closed but with the open mouth, it was easy to appreciate and measure. The small size of the lesion explains why it was not detected on prenatal MRI. Also, isolated cleft palate without cleft lip is difficult to diagnose although MRI is more helpful in this aspect than sonography in which shadowing from the surrounding facial bones is very disturbing. However, MR images are also disturbed by fetal motion between and during image acquisitions [14] and we also appreciated isolated bilateral cleft palate as late as in postnatal examination.
Case no. 22. a Fetal MRI. FIESTA/2D image, sagittal plane. The irregular contour of the palate is visible but impossible to assess as a tumor. b Postnatal MRI. 3D/T1-weighted image. The pedunculated nodule arising from the anterior part of the palate is well seen in this midsagittal section with the open mouth of the newborn. c Postnatal MRI. 3D/T1-weighted image after gadolinium injection, sagittal plane. With the mouth closed the nodule is no longer visible. One can only state that it does not enhance
As stated above it is not always easy to find the place of origin of the lesion on prenatal MRI. In case no. 18, we saw a hemangioma on the fetus’ head but in some sequences and planes it seemed to be intraosseous while in the others—subcutaneous. Postnatal MRI confirmed its superficial localization. In case no. 24, we did not see the connection between the inside of the skull and a small fluid-filled lesion beneath the skin of the head and, therefore, we did not establish the diagnosis of atretic encephalocele on prenatal MRI performed early, at 25 GW.
Finally in case no. 1 on postnatal MRI, we found uterine anomaly—unicornuate uterus. On prenatal examination uterus can be identified as a tiny structure between the urine-filled T2-hyperintense, T1-hypointense bladder and meconium-filled rectum which is T2-hypointense and T1-hyperintense at this gestational age (31 weeks). Uterine cavity can be seen only if it is filled with fluid as in case of hydrometrocolpos, otherwise it is invisible and impossible to assess (Fig. 5a). So any abnormal shape of the cavity can be diagnosed only after birth (Fig. 5b) [15].
We still face the problem with spatial resolution in fetal MRI. Postnatal MRI is not free of this problem but is definitely less affected by it. With the advent of two-dimensional fast imaging employing steady-state acquisition (FIESTA/2D) technique with parallel imaging, which was also used in this study, prenatal MRI achieved temporal resolution at less than half a second and quite high spatial resolution [16], however, it is still lower than that of neonatal MRI. By definition then, the number of details seen prenatally is limited as compared to postnatal visualization.
A recent study of the human fetus comparing signal-to-noise ratio (SNR) and image quality between 1.5 and 3.0 T MRI has shown higher image resolution and SNR, and—in approximately one-third of cases—superior tissue contrast and conspicuity in a 3.0 T scanner compared to 1.5 T MRI [17]. This might be the future for obtaining better images of fetuses with more anatomical and pathological details but for now most of fetal studies are performed on 1.5 T units and we do not see everything. Fortunately, most of the changes that went unnoticed on prenatal studies in our material had no significant impact on the management of newborn babies at this very moment of their lives. However, we do remember that many of these patients are syndromic ones and that the small undetected details cannot be underestimated as far as a final diagnosis is concerned.
A limitation of our study is that the sample size is relatively small. However, our study group was limited to patients who were referred for clinical fetal MRI first and then for MRI after birth. Continuously improving prenatal MRI and striving not to repeat this study postnatally, we have a limited number of cases in which clinicians consider MRI as indispensable after birth. This limitation could be overcome by performing postnatal MRI on all children who undergo fetal one but it would be unethical to sedate a neonate only for research purposes.
Another limitation is inhomogeneity of the material which consists of both neuroimaging and body imaging. However—in our opinion—being a limitation, it is also an advantage since—as mentioned above—the collected material reflects the whole spectrum of indications for which both prenatal and postnatal MRI is performed at present time.
Conclusions
The obvious advantage of imaging in the MR-compatible incubator is that a baby that has already been born is diagnosed, and not the tiny fetal body in a mother’s body, which improves image quality and provides opportunity to visualize tiny elements of anatomy as well as tiny pathological lesions, but still in most of our cases there was no added value of postnatal MRI as compared to prenatal one. This value lied in small details that could not have been noticed on prenatal MRI or required contrast medium administration to be noticed. On the other hand, MR examination performed with use of the dedicated neonatal coils in the MR-compatible incubator is a safe and reliable method of visualization of these small details with better spatial resolution thus helping to establish final diagnosis, treatment plan and prognosis.
References
Miller E, Ben-Sira L, Constantini S, Beni-Adani L (2006) Impact of prenatal magnetic resonance imaging on postnatal neurosurgical treatment. J Neurosurg 105(3 Suppl):203–209
Simon EM, Goldstein RB, Coakley FV, Filly RA, Broderick KC, Musci TJ et al (2000) Fast MR imaging of fetal CNS anomalies in utero. Am J Neuroradiol 21:1688–1698
Levine D, Barnes PD, Edelman RR (1999) Obstetric MR imaging. Radiology 211:609–617
Rona Z, Klebermass K, Cardona F, Czaba CD, Brugger PC, Weninger M et al (2010) Comparison of neonatal MRI examinations with and without an MR-compatible incubator: advantages in examination feasibility and clinical decision-making. Eur J Paediatr Neurol 14:410–417
Bekiesińska-Figatowska M, Helwich E, Rutkowska M, Stankiewicz J, Terczyńska I (2016) Magnetic resonance imaging of the neonates in the MR compatible incubator. Arch Med Sci 12(5) (in press)
Wagenvoort AM, Bekker MN, Go AT, Vandenbussche FP, van Buchem MA, Valk J et al (2000) Ultrafast scan magnetic resonance in prenatal diagnosis. Fetal Diagn Ther 15:364–372
Senapati GM, Levine D, Smith C, Estroff JA, Barnewolt CE, Robertson RL et al (2010) Frequency and cause of disagreements in imaging diagnosis in children with ventriculomegaly diagnosed prenatally. Ultrasound Obstet Gynecol 36:582–595
Glenn OA, Cuneo AA, Barkovich AJ, Hashemi Z, Bartha AI, Xu D (2012) Malformations of cortical development: diagnostic accuracy of fetal MR imaging. Radiology 263:843–855
Pacharn P, Kline-Fath B, Calvo-Garcia M, Linam LE, Rubio EI, Salisbury S et al (2013) Congenital lung lesions: prenatal MRI and postnatal findings. Pediatr Radiol 43:1136–1143
Brugger PC, Stuhr F, Lindner C, Prayer D (2006) Methods of fetal MR: beyond T2-weighted imaging. Eur J Radiol 57:172–181
Brugger PC (2012) MRI of the fetal face: anatomy of the eyes and orbits. J Pediatr Neuroradiol 1:161–170
Volpe P, Paladini D, Resta M, Stanziano A, Salvatore M, Quarantelli M et al (2006) Characteristics, associations and outcome of partial agenesis of the corpus callosum in the fetus. Ultrasound Obstet Gynecol 27:509–516
Mailáth-Pokorny M, Worda C, Krampl-Bettelheim E, Watzinger F, Brugger PC, Prayer D (2010) What does magnetic resonance imaging add to the prenatal ultrasound diagnosis of facial clefts? Ultrasound Obstet Gynecol 36:445–451
Kazan-Tannus JF, Levine D, McKenzie C, Lim KH, Cohen B, Farrar N et al (2005) Real-time magnetic resonance imaging aids prenatal diagnosis of isolated cleft palate. J Ultrasound Med 24:1533–1540
Bekiesińska-Figatowska M, Szkudlińska-Pawlak S, Romaniuk-Doroszewska A, Duczkowski M, Iwanowska B, Duczkowska A et al (2014) First experience with neonatal examinations with the use of MR-compatible incubator. Pol J Radiol 79:268–274
Guo WY, Ono S, Oi S, Shen SH, Wong TT, Chung HW et al (2006) Dynamic motion analysis of fetuses with central nervous system disorders by cine magnetic resonance imaging using fast imaging employing steady-state acquisition and parallel imaging: a preliminary result. J Neurosurg 105(2 Suppl):94–100
Krishnamurthy U, Neelavalli J, Mody S, Yeo L, Jella P, Saleem S et al (2015) MR imaging of the fetal brain at 1.5T and 3.0T field strengths: comparing specific absorption rate (SAR) and image quality. J Perinat Med 43:209–220
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study did not receive any funding.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from the parents or legal guardians of all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bekiesinska-Figatowska, M., Romaniuk-Doroszewska, A., Duczkowska, A. et al. Fetal MRI versus postnatal imaging in the MR-compatible incubator. Radiol med 121, 719–728 (2016). https://doi.org/10.1007/s11547-016-0649-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-016-0649-y