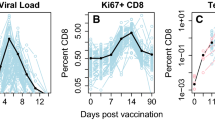
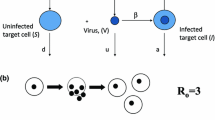
Abstract
The clonal expansion of T cells during an infection is tightly regulated to ensure an appropriate immune response against invading pathogens. Although experiments have mapped the trajectory from expansion to contraction, the interplay between mechanisms that control this response is not fully understood. Based on experimental data, we propose a model in which the dynamics of CD4+ T cell expansion is controlled through the interactions between T cells and antigen-presenting cells, where T cell stimulation is proportional to antigen availability, and antigen availability is regulated through downregulation of antigen by T cells. This antigen-dependent-feedback mechanism operates alongside an intrinsic reduction in cell proliferation rate that may also be responsible for slowing expansion. Our model can successfully predict T cell recruitment rates into division, expansion, and clonal burst size per cell when initial precursors are varied or when T cells are introduced late into an ongoing immune response. Importantly, the findings demonstrate that a feedback mechanism between T cells and antigen-presenting cells, along with a reduction in cell proliferation rate, can explain the ability of the immune system to adapt its response to variations in initial conditions or changes that occur later in the response, ensuring a robust yet controlled line of defence against pathogens.














Similar content being viewed by others
Availability of Data and Material
Not applicable.
References
Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR (2006) Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25(1):153–162
Bachmann MF, Kopf M (2002) Balancing protective immunity and immunopathology. Curr Opin Immunol 14(4):413–419
Bachmann MF, Oxenius A (2007) Interleukin 2: from immunostimulation to immunoregulation and back again. EMBO Rep 8(12):1142–1148
Badovinac VP, Tvinnereim AR, Harty JT (2000) Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-\(\gamma \). Science 290(5495):1354–1357
Beverley PC, Maini MK (2000) Differences in the regulation of CD4 and CD8 T-cell clones during immune responses. Philos Trans R Soc B 355(1395):401–406
Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R (2003) Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med 9(5):540–547
Borghans JA, Taams LS, Wauben MH, De Boer RJ (1999) Competition for antigenic sites during T cell proliferation: a mathematical interpretation of in vitro data. Proc Natl Acad Sci USA 96(19):10782–10787
den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mögling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K et al (2012) Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 36(2):288–297
De Boer RJ, Perelson AS (2013) Antigen-stimulated CD4 T cell expansion can be limited by their grazing of peptide-MHC complexes. J Immunol 190(11):5454–5458
Dhainaut M, Moser M (2014) Regulation of immune reactivity by intercellular transfer. Front Immunol 5:112
Diao J, Winter E, Cantin C, Chen W, Xu L, Kelvin D, Phillips J, Cattral MS (2006) In situ replication of immediate dendritic cell (DC) precursors contributes to conventional DC homeostasis in lymphoid tissue. J Immunol 176(12):7196–7206
Dowling MR, Kan A, Heinzel S, Marchingo JM, Hodgkin PD, Hawkins ED (2018) Regulatory T cells suppress effector T cell proliferation by limiting division destiny. Front Immunol 9:2461
Eriksson U, Kurrer M, Bingisser R, Eugster H, Saremaslani P, Follath F, Marsch S, Widmer U (2001a) Lethal autoimmune myocarditis in interferon-\(\gamma \) receptor-deficient mice: enhanced disease severity by impaired inducible nitric oxide synthase induction. Circulation 103(1):18–21
Eriksson U, Kurrer MO, Sebald W, Brombacher F, Kopf M (2001b) Dual role of the IL-12/IFN-\(\gamma \) axis in the development of autoimmune myocarditis: induction by IL-12 and protection by IFN-\(\gamma \). J Immunol 167(9):5464–5469
Furuta K, Ishido S, Roche PA (2012) Encounter with antigen-specific primed CD4 T cells promotes MHC class ii degradation in dendritic cells. Proc Natl Acad Sci USA 109(47):19380–19385
Ganusov VV, Milutinović D, De Boer RJ (2007) IL-2 regulates expansion of CD4+ T cell populations by affecting cell death: insights from modeling CFSE data. J Immunol 179(2):950–957
Gasteiger G, Kastenmuller W (2012) Foxp3+ regulatory T-cells and IL-2: the moirai of T-cell fates? Front Immunol 3:179
Homann D, Teyton L, Oldstone MB (2001) Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med 7(8):913
Hosking MP, Flynn CT, Whitton JL (2016) TCR independent suppression of CD8+ T cell cytokine production mediated by IFN\(\gamma \) in vivo. Virology 498:69–81
Jelley-Gibbs DM, Dibble JP, Filipson S, Haynes L, Kemp RA, Swain SL (2005) Repeated stimulation of CD4 effector T cells can limit their protective function. J Exp Med 201(7):1101–1112
Kamath AT, Henri S, Battye F, Tough DF, Shortman K (2002) Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood 100(5):1734–1741
Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P (2000) T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med 192(8):1105–1114
Lambrecht BN, Pauwels RA, Groth BFdS (2000) Induction of rapid T cell activation, division, and recirculation by intratracheal injection of dendritic cells in a TCR transgenic model. J Immunol 164(6):2937–2946
Liou HR, Myers JT, Barkauskas DS, Huang AY (2012) Intravital imaging of the mouse popliteal lymph node. J Vis Exp 60:e3720
Mayer A, Zhang Y, Perelson AS, Wingreen NS (2019) Regulation of t cell expansion by antigen presentation dynamics. Proc Natl Acad Sci USA 116(13):5914–5919
Mayerova D, Wang L, Bursch LS, Hogquist KA (2006) Conditioning of Langerhans cells induced by a primary CD8 T cell response to self-antigen in vivo. J Immunol 176(8):4658–4665
Morel PA, Faeder JR, Hawse WF, Miskov-Zivanov N (2014) Modeling the T cell immune response: a fascinating challenge. J Pharmacokinet Pharmacodyn 41(5):401–413
Obst R (2015) The timing of T cell priming and cycling. Front Immunol 6:563
Pappalardo F, Fichera E, Paparone N, Lombardo A, Pennisi M, Russo G, Leotta M, Pappalardo F, Pedretti A, De Fiore F et al (2016) A computational model to predict the immune system activation by citrus-derived vaccine adjuvants. Bioinformatics 32(17):2672–2680
Pennisi M, Russo G, Sgroi G, Bonaccorso A, Palumbo GAP, Fichera E, Mitra DK, Walker KB, Cardona PJ, Amat M et al (2019) Predicting the artificial immunity induced by Ruti® vaccine against tuberculosis using universal immune system simulator (UISS). BMC Bioinf 20(6):1–10
Quiel J, Caucheteux S, Laurence A, Singh NJ, Bocharov G, Ben-Sasson SZ, Grossman Z, Paul WE (2011) Antigen-stimulated CD4 T-cell expansion is inversely and log-linearly related to precursor number. Proc Natl Acad Sci USA 108(8):3312–3317
Rabenstein H, Behrendt AC, Ellwart JW, Naumann R, Horsch M, Beckers J, Obst R (2014) Differential kinetics of antigen dependency of CD4+ and CD8+ T cells. J Immunol 192(8):3507–3517
Schwartz RH (2003) T cell anergy. Annu Rev Immunol 21(1):305–334
Smith AL, Fazekas de St Groth B (2020) T cell competition profoundly reduces the effect of initial precursor frequency on the generation of CD4 T cell memory. bioRxiv https://doi.org/10.1101/2020.09.09.290627
Spencer AJ, Smith AL, de St Groth BF (2020) Antigen-specific competitive inhibition of CD4+ T cell recruitment into the primary immune response. bioRxiv
Tomura M, Hata A, Matsuoka S, Shand FH, Nakanishi Y, Ikebuchi R, Ueha S, Tsutsui H, Inaba K, Matsushima K et al (2014) Tracking and quantification of dendritic cell migration and antigen trafficking between the skin and lymph nodes. Sci Rep 4:6030
Van de Velde LA, Murray PJ (2016) Proliferating helper T cells require Rictor/mTORC2 complex to integrate signals from limiting environmental amino acids. J Biol Chem 291(50):25815–25822
Villarino AV, Tato CM, Stumhofer JS, Yao Z, Cui YK, Hennighausen L, O’Shea JJ, Hunter CA (2007) Helper T cell IL-2 production is limited by negative feedback and stat-dependent cytokine signals. J Exp Med 204(1):65–71
Yamamoto T, Hattori M, Yoshida T (2007) Induction of T-cell activation or anergy determined by the combination of intensity and duration of T-cell receptor stimulation, and sequential induction in an individual cell. Immunology 121(3):383–391
Yarke CA, Dalheimer SL, Zhang N, Catron DM, Jenkins MK, Mueller DL (2008) Proliferating CD4+ T cells undergo immediate growth arrest upon cessation of TCR signaling in vivo. J Immunol 180(1):156–162
Acknowledgements
The authors gratefully acknowledge support for this work through Australian Government Research Training Program Scholarship (PP), the Australian Research Council Discovery Project DP180101512 (PSK).
Funding
This study was funded by Australian Government Research Training Program Scholarship (PP), the Australian Research Council Discovery Project DP180101512 (PSK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest or competing interests.
Code Availability
Code available upon request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pooladvand, P., Kim, P.S. & Fazekas de St Groth, B. The Role of Antigen-Competitive Dynamics in Regulating the Immune Response. Bull Math Biol 83, 40 (2021). https://doi.org/10.1007/s11538-021-00867-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-021-00867-7