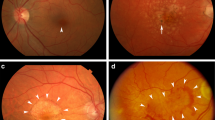
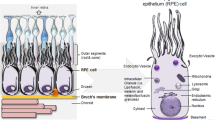
Abstract
In age-related macular degeneration (AMD), there is, in common with many other age-related diseases, the need to distinguish between changes in the ageing eye that lead to disease and those changes that are considered part of a healthy, ageing eye. Various studies investigating the multitude of mechanisms involved in the aetiology of AMD exist within the field of ophthalmology and related medical fields, yet many aspects of it remain poorly understood and only a limited number of therapies are available. A recent study relates drusen’s topographically cellular characteristics to the neural retina’s metabolic needs and associated cholesterol involvement within the retina. In particular, there is a need to fully understand the maintenance of cholesterol homeostasis in the retina to prevent normal ageing processes from being perturbed towards maculopathy. Here, we present an extensive review of the clinical and physiological features of the ageing retina, as well as mechanisms implicated in pathology, synthesised from a vast body of the published literature. We use this novel synthesis to construct a comprehensive process schematic, encompassing all key species and physiological processes such as nutrients, waste and lipoprotein management. We are therefore able to express these processes in a mathematical language via a comprehensive modelling framework, comprising a set of twenty-three equations spanning three distinct biological compartments. This very general modelling framework may now be adapted to more focused studies on individual mechanisms, processes or components underlying of the many facets of AMD. As an example of such a focused application, we conclude this article with a one-compartment, four-species model of the retinal pigment epithelium, which considers the parametric conditions under which either cholesterol homeostasis or unregulated accumulation of cholesterol may obtain in the ageing eye.








Similar content being viewed by others
Abbreviations
- AROS:
-
Anti-reactive oxygen species
- AMD:
-
Age-related macular degeneration
- BLamD:
-
Basal laminar deposit
- BLinD:
-
Basal linear deposit
- BLL:
-
Bilipid layer
- BM:
-
Bruch’s membrane
- CC:
-
Choriocappilaris
- CC BL:
-
Choriocappilaris basal lamina
- CNV:
-
Choroidal neovascularisation
- DHA:
-
Docohexaenoate acid
- EC:
-
Esterified cholesterol
- ECM:
-
Extracellular matrix
- EL:
-
Elastin layer
- GA:
-
Geographic atrophy
- HDL:
-
High-density lipoprotein
- ICL:
-
Inner collagenous layer
- LDL:
-
Low-density lipoprotein
- LDL-R:
-
Low-density lipoprotein receptor
- LSC:
-
Light-sensitive cells
- nAMD:
-
Neovascular AMD
- NR:
-
Neural retina
- OCL:
-
Outer collagenous layer
- POS:
-
Photoreceptor outer segments
- PR:
-
Photoreceptors
- RPE:
-
Retinal pigment epithelium
- RPE BL:
-
Retinal pigment epithelium basal lamina
- ROS:
-
Reactive oxygen species
- SDD:
-
Subretinal drusenoid deposit
- TG:
-
Triglycerides
- UC:
-
Unesterified cholesterol
- VEGF:
-
Vascular endothelial growth factor
- VLDL:
-
Very low-density lipoprotein
- TGF-\(\beta \) :
-
Transforming growth factor beta
- TIMP-3:
-
Tissue inhibitor of metalloproteinase-3
References
Araujo RP, McElwain DLS (2004) A history of the study of solid tumour growth: the contribution of mathematical modelling. Bull Math Biol 66(5):1039
Ardeljan D, Chan C (2013) Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retinal Eye Res 37:68–89
Ayala A, Munoz MF, Argucelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014:31
Beattie JR, Pawlak AM, Boulton ME, Zhang J, Monnier VM, McGarvey JJ, Stitt AW (2010) Multiplex analysis of age-related protein and lipid modifications in human Bruch’s membrane. FASEB J 24(12):4816–4824
Beatty S, Koh H, Phil M, Henson D, Boulton M (2000) The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 45(2):115–134
Bhutto I, Lutty G (2012) Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Asp Med 33(4):295–317
Booij JC, Baas DC, Beisekeeva J, Gorgels TGMF, Bergen AAB (2010) The dynamic nature of Bruch’s membrane. Prog Retinal Eye Res 29:1–18
Boulton ME (1991) Chapter 6 ageing of the retinal pigment epithelium. Prog Retinal Res 11:125–151
Brown MS, Goldstein JL (1975) Regulation of the activity of the low density lipoprotein receptor in human fibroblasts. Cell 6(3):307–316
Brown M, Goldstein J (1986) A receptor-mediated pathway for cholesterol homeostasis. Science 232(4746):34–47
Buch H (2005) Fourteen-year incidence of age-related maculopathy and cause-specific prevalence of visual impairment and blindness in a Caucasian population: the Copenhagen City Eye Study. Acta Ophthalmol Scand 83:5–32
Buschini E, Piras A, Nuzzi R, Vercelli A (2011) Age related macular degeneration and drusen: neuroinflammation in the retina. Prog Neurobiol 95(1):14–25
Chiras D, Kitsos G, Petersen MB, Skalidakis I, Kroupis C (2015) Oxidative stress in dry age-related macular degeneration and exfoliation syndrome. Crit Rev Clin Lab Sci 52(1):12–27
Covic A, Kanbay M, Lerma EV (2014) Dyslipidemias in kidney disease. Springer, New York
Curcio CA (2018a) Antecedents of soft drusen, the specific deposits of age-related macular degeneration, in the biology of human macula. Investig Ophthalmol Vis Sci 59(4):AMD182–AMD194
Curcio CA (2018b) Soft drusen in age-related macular degeneration: biology and Targeting via the oil spill strategies. Investig Ophthalmol Vis Sci 59(4):160–181
Curcio CA, Johnson M (2013) Chapter 20-structure, function, and pathology of Bruch’s membrane. In: Ryan SJ, Sadda SR, Hinton DR, Schachat AP, Wilkinson CP, Wiedemann P (eds) Retina, 5th edn. W.B. Saunders, London, pp 465–481
Curcio CA, Johnson M, Rudolf M, Huang J (2011) The oil spill in ageing Bruch membrane. Br J Ophthalmol 95(12):1638–1645
Curcio CA, Millican CL, Allen KA, Kalina RE (1993) Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Investig Ophthalmol Vis Sci 34(12):3278–3296
Curcio CA, Millican CL, Bailey T, Kruth HS (2001) Accumulation of cholesterol with age in human Bruch’s membrane. Investig Ophthalmol Vis Sci 42(1):265–274
Curcio CA, Presley JB, Malek G, Medeiros NE, Avery DV, Kruth HS (2005a) Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res 81(6):731–741
Curcio CA, Presley JB, Millican CL, Medeiros NE (2005b) Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp Eye Res 80(6):761–775
Dorey CK, Wu G, Ebenstein D, Garsd A, Weiter JJ (1989) Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci 30(8):1691–1699
Feinberg M (2019) Foundations of chemical reaction network theory. Springer, Cham
Ferris FL, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Sadda SR (2013) Clinical classification of age-related macular degeneration. Ophthalmology 120(4):844–851
Fliesler SJ (2010) Lipids and lipid metabolism in the eye. J Lipid Res 51(1):1–3
Fliesler SJ, Anderson RE (1983) Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res 22(2):79–131
Fliesler SJ, Bretillon L (2010) The ins and outs of cholesterol in the vertebrate retina. J Lipid Res 51(12):3399–3413
Gao H, Hollyfield J (1992) Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 33(1):1–17
Gatenby RA, Maini PK (2003) Mathematical oncology: cancer summed up. Nature 421(6921):321–321
Giachelli CM (2009) The emerging role of phosphate in vascular calcification. Kidney Int 75(9):890–897
Gordiyenko N, Campos M, Lee JW, Fariss RN, Sztein J, Rodriguez IR (2004) RPE cells internalize low-density lipoprotein (LDL) and oxidized LDL (oxLDL) in large quantities in vitro and in vivo. Invest Ophthalmol Vis Sci 45(8):2822–2829
Grassmann F, Mengelkamp J, Brandl C, Harsch S, Zimmermann ME, Linkohr B, Peters A, Heid IM, Palm C, Weber BHF (2018) A deep learning algorithm for prediction of age-related eye disease study severity scale for age-related macular degeneration from color fundus photography. Ophthalmology 125(9):1410–1420
Grossniklaus HE, Green WR (2004) Choroidal neovascularization. Am J Ophthalmol 137(3):496–503
Hageman GS, Luthert PJ, Chong NHV, Johnson LV, Anderson DH, Mullins RF (2001) An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retinal Eye Res 20(6):705–732
Handa J, Bowes Rickman C, Dick A, Gorin M, Miller J, Toth C, Ueffing M, Zarbin M, Farrer L (2019) A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat Commun 10(1):1–11
Hartnett ME, Weiter JJ, Garsd A, Jalkh AE (1992) Classification of retinal pigment epithelial detachments associated with drusen. Graefe’s Arch Clin Exp Ophthalmol 230(1):11–19
Hussain MA, Bhuiyan A, Luu CD, Theodore Smith R, Guymer RH, Ishikawa H, Schuman SJ, Ramamohanarao K (2018) Classification of healthy and diseased retina using SD-OCT imaging and Random Forest algorithm. PloS One 13(6):e0198281
Ioseliani O (2006) New developments in eye research. Nova biomedical. Nova Biomedical Books, New York
Jarrett SG, Boulton ME (2012) Consequences of oxidative stress in age-related macular degeneration. Mol Asp Med 33(4):399–417
Kent D, Sheridan C (2003) Choroidal neovascularization: a wound healing perspective. Mol Vis 9:747–755
Kijlstra A, Berendschot TTJM (2015) Age-related macular degeneration: a complementopathy? Ophthalmic Res 54(2):64–73
Kim J, Zhao H, Martinez J, Doggett T, Kolesnikov AV, Tang PH, Ablonczy Z, Chan C, Zhou Z, Green DR, Ferguson TA (2013) Noncanonical autophagy promotes the visual cycle. Cell 154(2):365–376
Krinsky NI, Cornwell DG, Oncley JL (1958) The transport of vitamin A and carotenoids in human plasma. Arch Biochem Biophys 73(1):233–246
Lambert NG, ElShelmani H, Singh MK, Mansergh FC, Wride MA, Padilla M, Keegan D, Hogg RE, Ambati BK (2016) Risk factors and biomarkers of age-related macular degeneration. Prog Retinal Eye Res 54:64–102
Liscurn L (2002) Chapter 15 cholesterol biosynthesis. New Compr Biochem 36:409–431
Luthert P (2010) Pathogenesis of age-related macular degeneration. In: Mini-symposium ocular pathology
Maguire MG, Bressler SB, Bressler NM, Alexander J, Hiner CJ, Javornik MS, Phillips D, Marsh MJ, Hawkins BS, Burgess DB, Chandra S, Klein ML, Orth DH, Stevens T, Fine L (1997) Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol 115(6):741–747
Marmor MF, Wolfensberger TW (1998) The retinal pigment epithelium. Oxford University Press, New York
Mast N, Reem R, Bederman I, Huang S, Dipatre PL, Bjorkhem I, Pikuleva IA (2011) Cholestenoic acid is an important elimination product of cholesterol in the retina: Comparison of retinal cholesterol metabolism with that in the brain. Invest Ophthalmol Vis Sci 52(1):594–603
McHarg S, Clark SJ, Day AJ, Bishop PN (2015) Age-related macular degeneration and the role of the complement system. Mol Immunol 67(1):43–50
Noske UM, Schmidt-Erfurth U, Meyer C, Diddens H (1998) Lipid metabolism in retinal pigment epithelium (RPE): a possible role of LDL receptors. Der Ophthalmol 95(12):814–819
Obana A, Sasano H, Okazaki S, Otsuki Y, Seto T, Gohto Y (2017) Evidence of carotenoid in surgically removed lamellar hole-associated epiretinal proliferation. Invest Ophtalmol Vis Sci 58(12):5157–5163
Oyster CW (1999) The Human Eye. Sinauer Associates, Inc, Sunderland
Pauleikhoff D, Harper CA, Marshall J (1990) Aging changes in Bruch’s membrane: a histochemical and morphological study. Ophthalmology 97:171–178
Pauleikhoff D, Zuels S, Sheraidah G, Marshall J, Wessing A, Bird A (1992) Correlation between biochemical composition and fluorescein binding of deposits in Bruch’s membrane. Ophthalmology 99(10):1548–1553
Pikuleva IA, Curcio CA (2014) Cholesterol in the retina: the best is yet to come. Progr Retinal Eye Res 41:64–89
Reichenbach A, Bringmann A (2013) New functions of Müller cells. Glia 61(5):651–678
Roberts PA, Gaffney EA, Luthert PJ, Foss AJE, Byrne HM (2016) Mathematical and computational models of the retina in health, development and disease. Progr Retinal Eye Res 53:48–69
Rodríguez IR, Larrayoz IM (2010) Cholesterol oxidation in the retina: implications of 7KCh formation in chronic inflammation and age-related macular degeneration. J Lipid Res 51(10):2847–2862
Rudolf M, Clark ME, Chimento MF, Li C, Medeiros NE, Curcio CA (2008) Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci 49(3):1200–1209
Sarks J, Sarks S, Killingsworth M (1988) Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Basingstoke) 2(5):552–577
Savic RM, Jonker DM, Kerbusch T, Karlsson MO (2007) Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 34(5):711–726
Schaal KB, Freund KB, Litts KM, Zhang Y, Messinger JD, Curcio CA (2015) Outer retinal tubulation in advanced age-related macular degeneration: optical coherence tomographic findings correspond to histology. RETINA 35(7):1339
Scholl HPN, Fleckenstein M, Charbel Issa P, Keilhauer C, Holz FG, Weber BHF (2007) An update on the genetics of age-related macular degeneration. Mol Vis 13:196–205
Shirinifard A, Glazier JA, Swat M, Gens JS, Family F, Jiang Y, Grossniklaus HE (2012) Adhesion failures determine the pattern of choroidal neovascularization in the eye: A computer simulation study. PloS Comput Biol 8(5):e1002440
Spaide RF, Armstrong D, Browne R (2003) Choroidal neovascularisation in age-related macular degeneration-what is the cause? Retina 23(5):595–614
Spraul CW, Lang GE, Grossniklaus HE, Lang GK (1999) Histologic and Morphometric Analysis of the Choroid, Bruch’s Membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol 44(1):S10–S32
Strauss O (2005) The retinal pigment epithelium in visual function. Phys Rev 85:845–881
Subczynski WK, Wisniewska A, Widomska J (2010) Location of macular xanthophylls in the most vulnerable regions of photoreceptor outer-segment membranes. Arch Biochem Biophys 504(1):61–66
Tabas I, Williams KJ, Borén J (2007) Subendothelial lipoprotein retention as the initiating process in atherosclerosis. Circulation 116(16):1832–1844
Thomas SE, Harrison EH (2016) Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J Lipid Res 57(10):1865–1878
Tserentsoodol N, Gordiyenko N, Pascual I, Lee J, Fliesler S, Rodriguez I (2006a) Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis 12:1319–1333
Tserentsoodol N, Sztein J, Campos M, Gordiyenko NV, Fariss RN, Lee JW, Fliesler SJ, Rodriguez IR (2006b) Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol Vis 12:1306–1318
van Lookeren Campagne M, LeCouter J, Yaspan BL, Ye W (2014) Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol 232(2):151–164
Wang L, Li C, Rudolf M, Belyaeva OV, Chung BH, Messinger JD, Kedishvili NY, Curcio CA (2009) Lipoprotein particles of intraocular origin in human Bruch membrane: an unusual lipid profile. Invest Ophthalmol Vis Sci Vis Sci 50(2):870–877
Wang ZL, Qiao BD, Li GX (2014) Bevacizumab cured age-related macular degeneration (AMD) via down-regulate TLR2 pathway abstract. Cent Eur J Biol 9(5):469–475
Wattis JAD, O’Malley B, Blackburn H, Pickersgill L, Panovska J, Byrne HM, Jackson KG (2008) Mathematical model for low density lipoprotein (LDL) endocytosis by hepatocytes. Bull Math Biol 70(8):2303
Wecker T, Ehlken C, Bühler A, Lange C, Agostini H, Böhringer D, Stahl A (2017) Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. Br J Ophthalmol 101(3):353–359
Wikimedia Commons (2018) File:retina layers.svg. Wikimedia Commons, the free media repository
Wikimedia Commons (2019) File:schematic diagram of the human eye en.svg. Wikimedia Commons, the free media repository
Williams KJ, Tabas I (1995) The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 15(5):551–561
Wolter JR, Falls HF (1962) Bilateral confluent drusen. Arch Ophthalmol 68(2):219–226
Xue Y, Shen SQ, Jui J, Rupp AC, Byrne LC, Hattar S, Flannery JG, Corbo JC, Kefalov VJ (2015) CRALBP supports the mammalian retinal visual cycle and cone vision. J Clin Invest 125(2):727–738
Zambon S, Orlando R, Sartore G, Bassix A, Manzato E, Crepaldi G (1995) The lipoprotein composition of plasma and ascitic fluid in liver cirrhosis. Eur J Clin Invest 25(3):143–148
Zarbin MA, Rosenfeld PJ (2010) Pathway-based therapies for age-related macular degeneration: an integrated survey of emerging treatment alternatives. RETINA 30(9):1350–1367
Zekavat SM, Lu J, Maugeais C, Mazer NA (2017) An in silico model of retinal cholesterol dynamics (RCD model): insights into the pathophysiology of dry AMD. J Lipid Res 58(7):1325–1337
Zheng W, Reem RE, Omarova S, Huang S, DiPatre PL, Charvet CD, Curcio CA, Pikuleva IA (2012) Spatial distribution of the pathways of cholesterol homeostasis in human retina. PLoS One 7(5):e37926
Zouache MA, Eames I, Luthert PJ (2015) Blood flow in the choriocapillaris. J Fluid Mech 774:37–66
Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y (2010) Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology 117(2):303–312
Acknowledgements
Robyn P. Araujo is the recipient of an Australian Research Council (ARC) Future Fellowship (project number FT190100645) funded by the Australian Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Scheepers, R., Pettet, G.J., van Heijster, P. et al. Cholesterol Regulation in Age-Related Macular Degeneration: A Framework for Mathematical Modelling of Drusen Biogenesis. Bull Math Biol 82, 135 (2020). https://doi.org/10.1007/s11538-020-00812-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-020-00812-0